Chronic pain & nociception
Connective tissue layers of the nerve
Peripheral nerves are made up of several hundred nerve fibers (axons). These bundles of axons are surrounded by connective tissue layers to form a fascicle. These fascicles are organised so they are juxtaposed to each other to form a nerve trunk. Sensory fibers: 4 and 10 mm diameter. Motor fibers: 2 and 6mm diameter (Teixeira 2016).
The nerve trunk lies, or is embedded, in a “surrounding tissue bed” comprised of muscles, tendons, fascia, blood vessels, or fat. The paraneurium (or mesoneurium) is composed of connective tissue that attaches the peripheral nerve to its surrounding nerve bed. The paraneurium directs strain along the course of the nerve by guiding the trajectory of peripheral nerves within their beds allowing for low-friction nerve “gliding”. This allows nerve regions that are accustomed to high strain to absorb mechanical loading, as, for instance, parts of the nerve that run over a mobile joint have to be less stiff and more compliant (Foran et al 2018). With elongation of the nerve bed, the paraneurium (or mesoneurium) glides the nerve towards the moving joint (‘convergence’) and consequently, when tension is relieved during joint motion, ‘divergence’ occurs whereby the paraneurium realigns the nerve along the shortened nerve bed by gliding it away from the moving joint (Topp & Boyd 2006).
The three layers of connective tissue that structurally support and regionally nourish the peripheral nerves are:
Epineurium: external layer. The epineurium envelopes several fascicles (bundles of axons, refer perineurium) that together form the nerve trunk (Teixeira 2016). If the nerve contains more than one fascicle, the epineurium may be divided into (i) the external epifascicular epineurium that surrounds the entire nerve trunk; (ii) the internal interfascicular epineurium that separates the nerve fascicles. There is abundant epineurial connective tissue in nerves that contain many fascicles allowing the connective tissue of the epineurium to facilitate the dispersion of a compressive force’s tendency to displace the nerve’s internal contents in transverse and longitudinal directions causing most damage to the axons and myelin where the shear forces are highest at the edges of the compressed zone (Topp & Boyd 2006). The epineurium also resists elongation (Teixeira 2016).
In a slackened position the epineurial collagen fibrils are relaxed with the interfascicular epineurium being loosely attached to the underlying perineurium. This allows one fascicle to slide independently of its adjacent fascicle.
The outermost tissue of the epifascicular epineurium is attached to the paraneurium (or mesoneurium) connective tissue. The density and strength of these attachments differ along the length of a nerve. Where the epifascicular epineurium is less adherent to the paraneurium this loose paraneurium connective tissue contains more adipose tissue to protect the nerve at sites of recurrent compression whilst facilitating transverse and longitudinal gliding of the nerve within the nerve bed. In contrast, the epifascicular epineurium is more tightly adherent to the paraneurium connective tissues where vessels enter or exit the nerve and where the nerve branches. Additionally, there are points at which a nerve may be firmly attached to an anatomical landmark, such as the attachment of the common peroneal nerve near the neck of the fibula (Topp & Boyd 2006).
Collagen fibers from the epineurium forms the outer layer of the dorsal root ganglia capusle (Aliyarbayova et al 2022) and then runs continuous with the outer most meningeal covering the dura mater at the subarachnoid angle (Weerasuriya & Mizisin 2011).
Perineurium (interfascicular connective tissue): middle layer. Made up of a sheath with several layers of perineural flat cells surrounded by a baseline membrane. The perineurium is arranged circumferentially to bundle together axons, Schwann cells, and endoneural components into a nerve fascicle. The perineurium acts as a blood-nerve barrier regulating the endoneuronal environment (Teixeira 2016).
In most peripheral nerves, motor and sensory fibers are intermingled. In general, nerve trunks are grouped into 4-10+ fascicles (bundles of axons surrounded by the perineurium). The perineurium continuously and individually covers these fascicles, that collectively form the nerve trunk, all the way down to the nerve terminal (Teixeira 2016).
However, axons do not remain in the same fascicle throughout their length and can move by means of connective tissue layers to other fascicles. The interchange of axons between fascicles help to minimise functional deficits following partial injury to the nerve, but, can also result in a wide distribution of macrophages cleaning up the debris from axons undergoing Wallerian degeneration after injury (Topp & Boyd 2006).
The perineurium divides into (i) the superficial layer of the perineurium forms the inner layer of the DRG capsule (Aliyarbayova et al 2022) and attaches to the middle meningeal covering, the arachnoid mater, at the subarachnoid angle. The arachnoid mater at this level forms the outer layer of the root sheath which then runs continuous with the pia mater at its emergence from the spinal cord; (ii) the inner layer of the perineurium continues to become the inner layer of the root sheath (Weerasuriya & Mizisin 2011) .
Endoneurium (intrafascicular connective tissue/Henle’s sheath): inner layer. Made up of connective tissue that individually surrounds intrafascicular Schwann cells-axon unit and fine fluid and fibrillar material as well as sheltering blood capillaries. The collagen fibrils of the endoneurium are closely packed around each Schwann cells-axon unit to form the supporting wall of the endoneurial tube. As the endoneurial tube encases the axon, its Schwann cell sheath, and the myelin (Menovsky 1999) they guide axonal regeneration (Teixeira 2016).
The axons of the peripheral nerve trunk have elasticity and a corrugated pathway so that they can be elongated up to 50% of their length before tension is directly transmitted to nerve tissue. Nerve roots have less connective tissue and are less corrugated than individual axons making them more vulnerable to mechanical distortion (Teixeira 2016).
Where the dorsal and ventral root attaches to the spinal cord the endoneurium of the spinal roots is continuous with the subarachnoid space providing continuity between CSF and endoneurial fluid (Weerasuriya & Mizisin 2011). The arachnoid mater that forms the nerve sheath of the intrathecal spinal roots is elastic, whereas the continuing epineurium that ensheathes the spinal nerves and more distal nerve trunks is relatively inelastic (refer ‘epineurium’). This point of stress between the elastic nerve root sheath and inelastic covering of the spinal nerves and trunks may create endoneurial inflammatory oedema stretching the relatively inelastic epi-perineurium beyond the limit of its compliance. This results in constriction of the transperineurial vessels accompanied by endoneurial ischaemia and nerve conduction failure (Berciano 2018).
The different type of Schwann Cells (a glial cell) include:
Myelinating Schwann cells. The plasma membrane of a Schwann cell is wrapped tightly multiple times around the axon forming the myelin sheath of each myelinated axon. Thus, a single Schwann cell envelops a single myelinated axon producing non-conductive myelin in large diameter myelinated axons which increases conduction speed. Each Schwann cell covers 300 to 2000mm of the axon length, being longer in larger axons.
The uninsulated 1mm gaps in the myelin sheath, the points of separation between the myelinating Schwann cells, are called nodes of Ranvier. The nodes of Ranvier are enriched with ion channels that enables them to exchange ions to regenerate action potentials. Unmyelinated axons are enveloped by Schwann cell cytoplasm and plasma membrane but do not have multiple wrappings of Schwann cell plasma membrane. A single Schwann cell may envelop several unmyelinated axons (Topp & Boyd 2006).
Myelinating Schwann cells can be triggered by a peripheral nerve injury to change function and aid regeneration by increasing the production of growth factors. However, as most Schwann cells are axonal sheaths located outside the DRG the specific changes that occur post-injury in the Schwann cells to effect nociception in the DRG is questionable (Martin et al 2019).
Satellite glial cells (nonmyelin-forming Schwann Cells). An accumulation of cell bodies from thousands of somatic, sympathetic, and parasympathetic sensory neurones, (up to 70,000) are located in the DRG, trigeminal ganglia and other ganglia associated with the cranial nerves. The cell bodies are surrounded by envelopes of satellite glial cells (SGCs) that form adhesive, tight, and gap junctions, are enriched for cytokine and interleukin signalling expressing proinflammatory molecules, buffers potassium concentrations in the ganglia and regulates gliosecretion in astrocytes after injury. Therefore, SGCs modify the microenvironment of neurons by regulating the uptake and release of molecules that supply nutrients to the surrounding neurons having a trophic function, influence synaptic transmission and have some structural function.
SGCs proliferate and alter after a peripheral nerve injury (Martin et al 2019):
(i). Their adhesive, tight gap junction temporarily becomes bigger.
(ii). The number of activated SGCs connected with SGCs of other neurones increases forming a ‘glial cohesion’ whereby more cell bodies in the ganglia share a common glial sheath; (iii) the SGCs loose their potassium regulatory functions elevating potassium concentrations in the ganglia, which, within itself, and by down-regulating the SGC removal of glutamate from the cell bodies (as well as increasing importation of glutamate) increases neuronal excitability (Krock et al 2023). Glutamate is the primary nocicpetive transmitter in the DRG (Haberberger et al 2019).
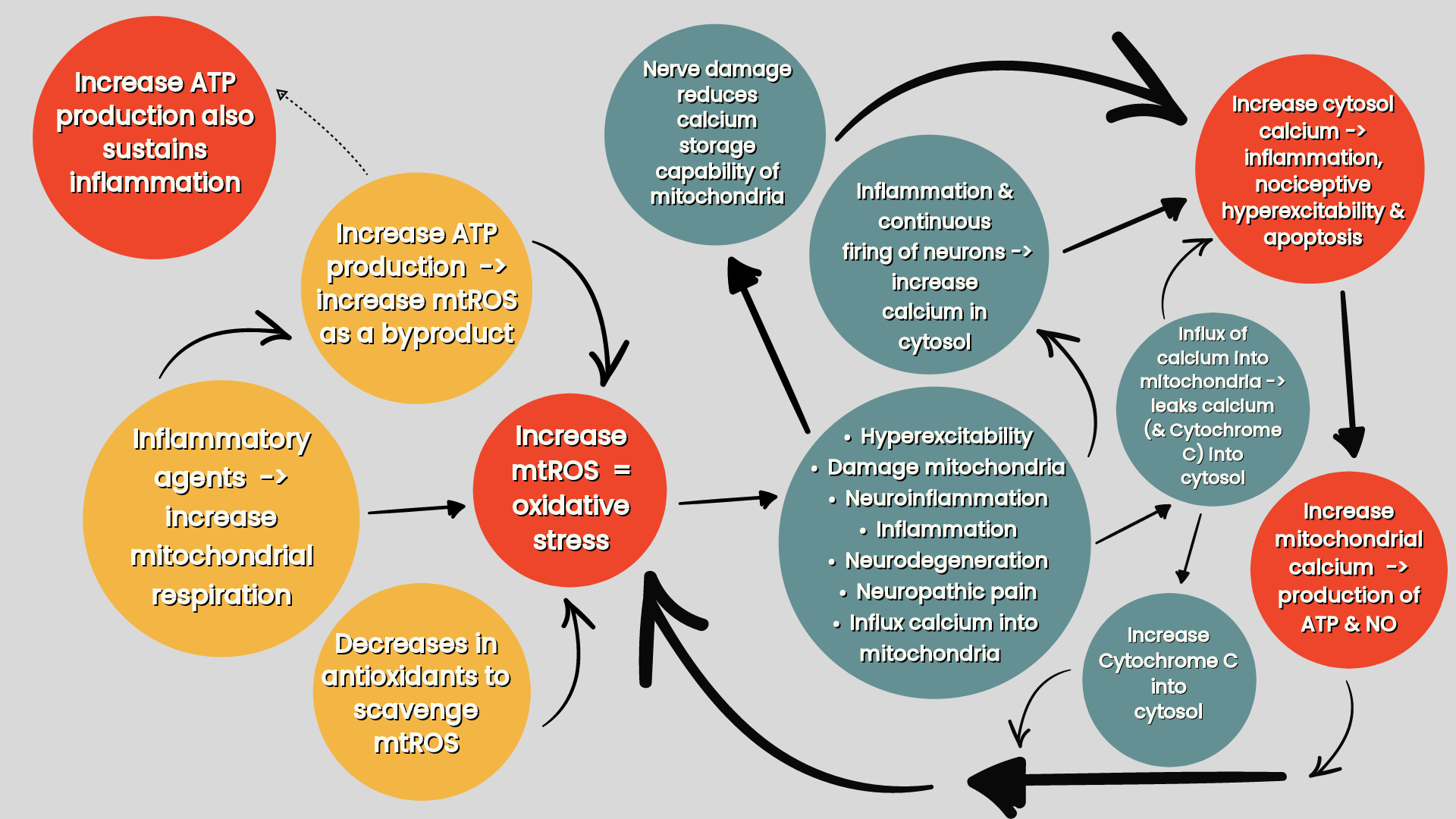
(iv) the release inflammatory transmitters induces chemical signals which generate electrical signals that get transmited to the DRG or trigeminal ganglion e.g. pain —> increase nerve impulses to DRG —> SGCs and neurones release large quantities of ATP (& proinflammatory cytokines) —> increase intracellular calcium concentration —> increase neuronal excitability, sensitivity and activation of SGCs (refer ‘Mitochondria & sensory processing’) (Krock et al 2023).
This makes SCGs central to the inflammatory process, and hence, increased DRG neuronal cell death (Martin et al 2019) and by how this influences neuronal excitability, the consequential development and maintenance of chronic pain (Andreeva et al 2022) (refer ‘Compression (or systemic inflammation) —> neuroinflammation —> neural oedema —> impaired axoplasmic flow. Neuroinflammation & impaired axoplasmic flow —> axonal mechanosensitivity —> peripheral sensitisation —> central sensitisation’). As DRG size increases from C1-8 and from L1-5 but decreases from S1 to S4 (with a very large ganglion at S1) could the correlation between DRG size and number of neurones contained within the DRG (Haberberger et al 2019) reflect severity of symptomology in resposne to pathological changes?
The central role of SGCs to the inflammatory process in the DRG can be seen in fibromyalgia. The association between IgG binding to SGCs (the autoantibody ‘anti-SGC IgG’) in the DRG and nociceptor hyperactivity and reduced intraepidermal nerve fiber density has been documented in fibromyalgia patients (Krock et al 2023). These IgGs binding to SCGs in the DRG produce spontaneous pain by, potentially, increasing spontaneous activity in, and sensitising nociceptive afferents. Even though these IgGs don’t bind to any other cell in the spinal cord or brain and are not involved in central mechanisms of evoked pain that can be seen in fibromyalgia patients, high levels of these ‘anti-SCG IgG’ autoantibodies are associated with low levels of certain metabolites in the thalamus and rostral anterior cingulate cortex that is associated with fibromyalgia disease severity (Fanton et al 2023).
In sensory nerves even though there are five thousand to nine thousand myelinated fibers (varies to individuals), there are two to six times more unmyelinated fibers. Schwann Cells-axon unit diameters are 0.5- 3.0mm < 1.5mm, increase myelin —> increase Schwann Cells-axon unit diameter (Teixeira 2016).
Group C-fibers are unmyelinated and have low conduction speed (Group A and B are myelinated and have high conduction speed). Schwann Cells bundle these C-fiber axons close together but stops them touching by squeezing the cytoplasm between the axons (Teixeira 2016).
Vascular supply to the nerve
Blood supply to the dorsal root ganglia
The DRG doesn’t just contain the neuronal cell bodies of primary sensory neurones, their axonal hillock, initial segment (enveloped by SCGs) and its continuing peripheral axon (enveloped by the meninges including the dura mater that is continuous with ganglion sheath), but small blood vessels. These blood vessels deliver blood and oxygen to satisfy the extensive energy demand of sensory neurones that have long processes, and so have a high-energy demand that is critical for maintaining the production and transport of receptors, ion channels, cytoskeletal and transport proteins (Haberberger 2019).
This extensive network of arterioles and capillaries that interface with the sensory neurones and blood vessels in DRG is unique making the DRGs highly vascularised. The fenestarted capillaries allow large quantities of blood borne molecules circulating in the vascular system to directly enter the DRG and interact with neuronal cells and non-neuronal cells including the immune cells that are contained within DRG that consist of macrophages (one of the key cells that modulate pain signalling), T-lymphocytes and B-lymphocytes (Haberberger 2019). The downside to these fenestrated capillaries allowing large quantities of blood molecules to enter and irrigate DRG is that antigens, infecting agents or immune cells can easily enter the DRG such as the Herpes virus and IgGs which bind to SGCs in the DRG (the autoantibody ‘anti-SGC IgG’) (Krock et al 2023) producing pain, rather than evoked pain in fibromyalgia (Fanton et al 2023).
This vascular organisation allows for an efficient exchange of nutrients, gases, and metabolites in the DRG and has two interconnected deep and superficial arterial plexuses that has a significantly higher perfusion in women compared to men (Haberberger 2019).
Blood supply to the nerve trunk
Nerve trunks are perfused by blood vessels originating from the collaterals of adjacent arteries. Vascular and inflammatory responses in the nerve accompany fibrotic tissue in the nerve’s surrounding connective tissue (Kitamura et al 1995).
The peripheral nerves have an extrinsic and intrinsic vascular supply:
Extrinsic vascular system: includes coiled segmental regional vessels that run in the loose "adventitia" or along the paraneurium (or mesoneurium) surrounding the nerve. The looseness of this “adventitia” or paraneurium allows the nerve trunk considerable mobility in its bed (Kitamura et al 1995).
Intrinsic vascular system: comprises the vasonervorum. It includes numerous vascular plexa in the epineurium, perineurium, and endoneurium (Kitamura et al 1995). After perforating the epineurium, the arteries divide into epineurial arterioles that form an anastomotic network that run within the epifascicular and interfascicular epineurium. Perforating arterioles cross the perineurium carrying with them a short sleeve of perineurial cells into the fascicle. Perineurial arterioles have poorly developed smooth muscles which limits their ability to regulate intrafascicular blood flow. Within the intrafascicular endoneurium, arterioles become large-diameter capillaries that form wide anastomotic longitudinal chains allowing blood to flow in either direction. Although the endothelial cells of endoneurial capillaries are connected by tight junctions to form a tight blood-nerve barrier (Topp & Boyd 2006), which selectively regulates the transfer of circulating substances inside the endoneurium (Teixeira 2016), due to the absence of astrocytes, this physical barrier is considered to be looser than the BBB in the CNS (Li et al 2023).
Vascular supply and fibrosis of the nerve
When the peripheral nerves are stretched, the surrounding blood vessels are extended, resulting in decreased blood flow within the nerve bundle. How this effects the extrinsic and intrinsic blood flow varies (Kitamura et al 1995):
Extrinsic system: blood flow decreases sharply in the presence of relatively small amount of traction and then decreases in a slow linear fashion with further increases in traction.
Intrinsic system: on the other hand, the blood flow through the intrinsic system shows a slower linear decrease in response to traction.
The difference in blood flow between the two systems can be explained by the biomechanics of the nerve in response to stretch. If stretching the nerve stretches the blood vessels then stretching the blood vessels will result in less circulation. In such a case the area stretched the least and has the better circulation should redirect blood to the area that is stretched the most and has the worst circulation (Kitamura et al 1995).
When a nerve is stretched blood is redirected from the less elongated portion i.e. the extrinsic system (paraneurium), as it flows through the loose “adventitia”, to the more elongated portion i.e. the intrinsic system (epi-, peri- & endoneurium), that gets pulled tighter (Kitamura et al 1995).
The sharp decrease in the blood flow through the extrinsic system can lead to local elevation of vascular permeability and oedema in the internal system. With repeated stretching over a two week span this has been shown to lead to hypertrophic connective tissue in the paraneurium and nerve bed and vascularisation of the epineurium and the surrounding tissue (Kitamura et al 1995). Could this cause a cascade that results in a further decrease movement by paraneural fibrosis, and in turn vascular-fibrotic changes in the nerve?
In response to peripheral nerve injury, as opposed to repeated stretch, fibrotic cells enveloping blood vessels in all three connective tissue layers of the nerve compartmentalise the endoneurium into many small fascicles. This can result in a persistent hypoxic state of the nerve, including microvascular dysfunction, endoneurial fibrosis, and increased metabolic loads (Lim et al 2015).
Could epiperineural fibrosis, causing a dynamic intraneural compression of the intact axons and their bundles, result in nociceptive sensitisation (refer ‘Intermittent (dynamic) partial compressive nerve lesion as an essential cause of persistent sensitisation and pain’ (Macionis 2023)? Could also endoneurial fibrosis restrict the internal mobility of the nerve bundle again creating a sensitisation? This type of fibrosis is seen around the brachial plexus during operations on patients with traumatic thoracic outlet syndrome (Chuang et al 2016).
Nervi nervorum
Nerve trunks are mechanosensitive because their connective tissue has afferents that participate in mechanoreception. Peripheral nerves are innervated by the nervi vasorum (that innervate the epineural blood vessels) and the nervi nervorum. The nervi nervorum are made up of small (unmyelinated or thin myelinated) fibers in the epineurium, perineurium and endoneurium (including encapsulated Pacini corpuscles in the endoneurium) (Teixeira 2016).
There are four distinct regions of peri- and non-perivascular innervation of the nerve trunks (Teixeira 2016):
Perivascular innervation of extraneural arteries and veins.
Perivascular innervation of intraneural blood vessels: vasa nervorum.
Non-perivascular fibers, the nervi nervorum, originates from the nerve trunk and perivascular plexus (vasa nervorum) and is distributed by epineural, perineural and endoneural connective tissues.
Intrafascicular fibers has a major adrenergic component, particularly in the sciatic nerve.
Anatomical peculiarities of the nervi nervorum and vaso nervorum makes them vulnerable to stretch and damage. Injury to the nervi nervorum can result in nerve sprouting and the development of neuromas. The result can be neuronal hyperreactivity (Teixeira 2016), and as a result of this C-fibers activity the release of angiogenic inflammatory substances in the form of increase in peripheral blood flow attributable to the release of vasoactive neurotransmitters from peripheral nociceptor terminals (Wu et al 2002) targeted at the vasa nervorum. These inflammatory substances can causes neurogenic inflammation and regional oedema precipitating a vicious compression-inflammation cycle (Teixeira 2016). Therefore, the nervi nervorum is both nociceptive and nocifensive as it responds to damaging stimuli by contributing to local inflammation in order to help defend and maintain the nerve’s local environment (Bove 2008). The ectopic impulses generated by the nervi nervorum, as a consequence of this injury cycle, can give local as well as referred pain (Teixeira 2016).
Neurogenic inflammation generated by nervi nervorum propagates mechanosensitivity along the nerve trunk distal from the injury site. Nervi nervorum sensitisation accounts for almost 50% of presentations of pain and paraesthesia that does not correspond to regions of the affected nerve root (Teixeira 2016).
However, when entrapment neuropathies lead to the structural degeneration (as opposed to sensitisation) of the small unmyelinated C-fibers that form the nerve nervorum there is decreased firing on neurodynamic testing. This may account for the >54% of patients with CTS having negative neurodynamic tests and approximately 58% of patients with lumbosacral radiculopathy have a negative SLR (Baselgia et al 2017). It should also be remembered that pathologies casuing demyelination of the nerve, whilst causing spurious activity in axons, does not necessarily affect the nociceptors as they are not myelinated (Bove 2008). As well as nocicepetive function tested on neurodynamic testing C-fibers can also be tested by pin prick and hot/cold sensation.
Biomechanics of the nerve
Elongation of the nerve
On initial stretch to a resting nerve, the nerve lengthens markedly so that the wavy connective tissue and axons in the endoneurial core straightens out. As further stretch is applied the nerve elongates (‘creeps’) at a steady rate (Topp & Boyd 2006).
Elongation of a nerve reduces it cross-sectional area, known as transverse contraction. This results in an increased pressure in the endoneurial core which, in turn, resists further transverse contraction and contributes to the stiffness of the nerve when under stretch (< centre of the elongated segment). The interface between the surrounding inner most layer of the perineurium, and the pressurised endoneurial core which it constrains, provides some minimal resistance to elongation of the nerve (Topp & Boyd 2006).
With elongation of the nerve bed, the paraneurium (or mesoneurium) glides the nerve towards the moving joint (‘convergence’) (refer ‘Connective tissue layers of the nerve’). For example, elbow extension elongates the median nerve as it aligns itself along the lengthened nerve bed. The paraneurium does this by gliding the nerve segment in the arm distally towards the elbow and the nerve segment in the forearm proximally towards the elbow. Consequently, when tension is relieved during joint motion ‘divergence’ occurs whereby, for example, during elbow flexion the median nerve slackens back off as the paraneurium realigns the nerve along the shortened nerve bed by gliding it away from the moving joint. With limb movement, nerve excursion occurs first in the nerve segment immediately adjacent to the moving joint, where the magnitude of excursion is greatest, and then as the limb continues to move, excursion occurs at nerve segments that are progressively more distant from the moving joint (Topp & Boyd 2006).
With increasing stretch stages of structural separation occurs: (i) in the interface between the perineurim and endoneurial compartment; (ii) between the axons and connective tissues in the endoneurial core; (iii) in the cells and connective tissues of the perineurial and epineurial sheath. Therefore, damage to axons in the endoneurial core may occur long before visible damage to the epineurium (Topp & Boyd 2006).
Factors influencing nerve stiffness
The paraneurium directs strain along the course of the nerve by guiding the trajectory of peripheral nerves within their beds allowing for low-friction nerve “gliding”. This allows nerve regions that are accustomed to high strain (i.e. areas of the nerve that are less stiff and highly compliant) to absorb this mechanical loading (Foran et al 2018). Factors influencing nerve stiffness are (Topp & Boyd 2006):
There is greater nerve compliance and less stiffness in nerve segments that cross joints, rather than in segments that do not cross joints.
There is less compliance and greater stiffness at points where the nerve is firmly attached to an anatomical structures, such as the attachment of the common peroneal nerve near the neck of the fibula.
Severing nerve branches or vessels results in increased compliance and decreased stiffness of the nerve. The epineurium is more tightly adherent to the paraneural connective tissues where vessels enter or exit the nerve and where the nerve branches.
The nerve shows less compliance and greater stiffness when the nerve is elongated rapidly rather than slowly. However, as this stretch is maintained there is a reduction in the tension in the nerve. A majority of this relaxation occurs in the first 20 minutes of fixed elongation as the nerve tissue elongates gradually (“creeps”) under the sustained load in order to provide protection in postures whereby the nerves are lengthened under prolonged tensile strain.
Chronic pain: nociception
Chronic pain, i.e. pain that lasts more than three months, is traditionally thought to be principally due to central sensitisation, which is driven by neuroinflammation in many chronic pain conditions. Although central mechanisms of sensitisation are important, they cannot completely account for such chronic pain features as chronicity, spontaneity, and, most importantly, individual variability in occurrence. The evidence supporting central sensitisation (autonomous continuing input-independent pain) as a source of chronic pain is relatively scanty and is outweighed by contrary evidence (Macionis 2023).
So where can nociceptive input come from when there is no obvious pathology? Could the answer be because the paraneural tissue damage, i.e. noxious input, resulting in stimulation of nociceptive fibers experienced in acute pain, persist and so sensitise the nociceptive fibers to become chronic pain? Peripheral nerve injury, although associated with neuropathic pain, is recognised as a cause of chronic pain, e.g. CRPS type I, fibromyalgia, and numerous small fiber neuropathy (SFN) conditions (Macionis 2023).
Three types of pain may manifest when a peripheral nerve is damaged (Teixeira 2016):
Pain at the site of nerve trunk injury. Described as stabbing or tender. This is attributed to increased activity of abnormal nociceptors that have been chemically or mechanically sensitised (Teixeira 2016). After a few days of inflammation axons become capable of generating autonomous (ectopic) action potentials, without stimulation from their terminal receptive fields. For instance, instead of generating an action potential from touching the skin an inflamed axon will just spontaneously fire off an action potential. The peak of these ectopic impulses occur four to seven days after the initial insult, and then decreases to insignificance or heals within two months (Bove 2008).
Disestesic and piercing pain. Described as burning, smarting or tingling or electricity. Disestesic pain is a consequence of regeneration of nociceptors which become abnormally excitable from abnormal activation of nociceptive afferents in the nervi nervorum.
Paroxysms. Described as a sudden attack of shock, stabbing, jumping or a deep pain following the nerve trunk and can be accompanied by allodynia. Symptoms are localised in the distribution of a sensory, or mixed nerve, and correspond to areas of sensory deficit and allodynia. These signs and symptoms are attributed to hyperactivity of mechanically and chemically sensitised nociceptors of the nervi nervorum whereby the pain is worsened with movement, stretching or palpation of the nerve trunk.
Compression (or systemic inflammation) —> neuroinflammation —> neural oedema —> impaired axoplasmic flow. Neuroinflammation & impaired axoplasmic flow —> axonal mechanosensitivity —> peripheral sensitisation —> central sensitisation
The peripheral nerves are very susceptible to overload, nerve function can be disturbed by pressures as low as 30 millimeters of mercury with ongoing neuronal activity developing after three weeks of repetitive overuse (Macionis 2023).
Paraneural tissue injury and inflammation < peripheral nerve lesion (e.g. nerve compression) —> neural oedema —> impairs axoplasmic transport. Neuroinflammation and impaired axoplasmic transport leads to peripheral sensitisation of afferent fibers (A- and C-type) causing hyperexcitability and consequential hyperactivity of the PNS (Macionis 2023). This is seen, for example, in peripheral nerve trunk pain, where the small fibers of the nociceptive nervi nervorum become sensitised (Teixeira 2016). As well as sensitising afferent nociceptive nerves these inflammatory mediators also trigger damage to sympathetic nerve fibers in the joints contributing to inflammatory arthritic pain (Silva et al 2022).
This axonal mechanosensitivity may explain why the most prevalent anatomic sites of chronic pain are those with high degrees of mobility, are subject to mechanical overload and are tightly interconnected with the spinal plexuses e.g. the extremities and lower back (Macionis 2023). This is because with movement, nerve excursion in these mechanosensitive nerves occurs first in the nerve segment immediately adjacent to the moving joint, where the magnitude of excursion is greatest, and then as the joint continues to move, excursion occurs at nerve segments that are progressively more distant from the moving joint (Topp & Boyd 2006). Endoneurial oedema, which can cause nerve conduction block, may quickly develop because of repetitive compression at these mobile sites where these mechanosensitive nerves have to be more compliant and less stiff, and maybe an ectopic cause of repetitive strain injury (e.g. nonspecific arm pain) (Macionis 2023). This may contribute to C2 DRG symptomology as the unusual relationship of the DRG being posterior to the C1-2 facet joint (Haberberger et al 2019), and the encasing of the C2 DRG, either within the posterior atlanto-axial ligament, or within an investing fascia that holds the C2 DRG, roots and spinal nerves against the capsule of the C1-2 facet joint can produce (Bogduk 1981) permits substantial mobility of a mechanosensitive C2 DRG.
Whilst axonal mechanosensitivity can be induced by atraumatic non-inflammatory disruption to axoplasmic flow, spontaneous, ongoing nociceptor activity can only occur with a focal neuritis (neuroinflammation). However, in reality neuroinflammation is likely to accompany all nerve injuries (Macionis 2023).
Nociceptive sensitisation, from the neuroinflammatory response to a neuritis (arising from both neural and non-neural tissue), and ion channel mutations causing a reduced activation threshold of ion channels, creates ongoing (spontaneous) activity and mechanical sensitivity of primary sensory fibers. This can account for tissue hypersensitivity in the form of disproportionate pain to noxious stimuli (hyperalgesia) and to non-noxious stimuli (allodynia) (Macionis 2023).
These ectopic neural impulses (i.e. action potentials not arising normally from nerve ending receptors) has traditionally been classified as being generated from nerve injuries stimulating sensory fibers and radiculopathies stimulating neuron cell bodies. However, recently, it has been found that these ectopic, spontaneous impulses in chronic nerve constriction is generated by the initial segment.
Normally sensory DRG neurones generate action potentials at the peripheral ending and then the axon initial segment and axon hillock, that are located in the DRG, determines the passage of action potentials to the central terminals of the sensory neurones in the dorsal horn (Haberberger et al 2023). However, nerve injury-induced DRG inflammatory changes, and consequential activation of SCGs, produces sensory neuron hyperexcitability (Macionis 2023) and spontaneous generation of action potentials in the sensory nerve at its axon initial segment (Haberberger et al 2023).
As well as containing the cell bodies and the axon initial segment encased by SCGs, that extends to a peripheral axon whose epineurium is encased by the meninges, including a thick layer of dura mater that is continuous with ganglion sheath, the DRG also contains an extensive network of arterioles and capillaries. These capillaries are fenestrated so that many inflammatory molecules such as macrophages (one of the key cells that modulate pain signalling), T-lymphocytes and B-lymphocytes can directly enter the DRG and interact with neuronal cells and non-neuronal cells (Haberberger et al 2019). Whilst the DRG contains low numbers of nerve endings from neurones that originate from outside the DRG, in response to nerve injury these numbers increase causing a sprouting of sympathetic and sensory fibres into the DRG (Haberberger et al 2023) that may potentially complicate the inflammatory changes and generation of ectopic impulses in the DRG.
Whilst acute inflammation is required to clear the cellular debris and improve the environment for regeneration after two months of chronic inflammatory changes from a peripheral nerve injury up to 35–40% of DRG neurones die (Martin et al 2019). The hyperactivity and spontaneous activity in the surviving afferent DRG neurones generate spontaneous, ectopic impulses (Macionis 2023).
Either from chronic inflammatory changes or cellular death of the DRG neurones the ongoing ectopic activity and axonal mechanosensitivity of afferent fibers (both of the A- and C-type) is induced by persistent intermittent (dynamic) partial proximal paraneural and intraneural compression (e.g. myofascial tension) —> peripheral nerve damage. This intermittent, partial injury to the paraneural tissues producing focal neuroinflammation results in a loss of DRG neurones and DRG neuron body sensitisation accounting for ongoing activity in nociceptive neurons (nococieptive sensitisation) (Macionis 2023).
It is crucial that the injury to the paraneural tissues is intermittent and partial as more extreme degrees of nerve compression results in axonal death of neural tissue which reduces nerve function (e.g. loss of motor power and anaesthesia), and creates a discontinuity between the axon and the nervi nervorum reducing its mechanosensitivity (Macionis 2023).
This DRG neuron hyperexcitability and consequential hyperactivity, in turn, triggers a hyperexcitability and consequential hyperactivity of the CNS. When these hyperexcitable and hyperactive action potentials in the CNS are processed in the brain cortex as persistent pain, and an abnormal extraterritorial hypersensitivity of non-lesioned tissue ensues, the patients has progressed to a state of central sensitisation (Macionis 2023).
Intermittent (dynamic) partial compressive nerve lesion as an essential cause of persistent sensitisation and pain
Extreme degree of distal nerve compression, in contrast to that of a moderate grade, does not result in peripheral tissue hypersensitivity. This is because extreme degrees of nerve compression triggers axonal degeneration resulting in a discontinuity between the axon and the nervi nervorum, a complete resolution of distal focal inflammation and the subsiding of ectopic impulses (Macionis 2023).
Inflammatory changes in the DRG and spinal cord are more pronounced after chronic nerve constriction, rather than a complete transection of the nerve. Therefore, it is persistent intermittent (dynamic) partial proximal peripheral nerve damage (e.g. myofascial tension) with its resultant continuing, chronic, focal neuroinflammation that causes a consequential hyperexcitability of the DRGn (Macionis 2023).
Although healed (fibrotic) neural lesions are physiologically silent, and so consequently cannot provide nociceptive input, scarred paraneural tissue can cause intermittent neural damage by restricting mobility of nerves. Via this mechanism, intraneural fibrosis of peripheral nerves may also result in dynamic intraneural compression of intact axons and their fasicicles (Macionis 2023) (refer to ‘vascular supply of the nerve; vasculat supply and fibrosis of the nerve’).
Intact axons and bundles of axons are relatively mobile; axons in the peripheral nerve trunk are elastic and have a corrugated pathway so they can elongate up to 50% of their length before tension is directly transmitted to nerve tissue (Teixeira 2016) and loose connective tissue allows movement of axons and fascicles in respect to each other. Therefore, any epiperineural scarring surrounding these healthy, mobile axons and fascicles may lead to an internal, dynamic, entrapment during motion. Whilst this type of dynamic intraneural nerve compression may produce only subtle nociceptive input, it may be sufficient to maintain central sensitisation via long-term potentiation (Macionis 2023). This impairment of the nociceptive inhibitory system being associated with central sensitisation is illustrated in migraine patients who exhibit more distal, broader, sensitivity in the tibialis anterior and median nerve (Del-Blanco-Muñiz et al 2023).
Nerve compression may intermittently be produced not only by neuromuscular but also by neurovascular mechanisms as seen, for example, in trigeminal neuralgia. This neurovascular mechanism, involving reflexive vasodilation, vasoconstriction and transient oedema, may be responsible for both intraneural and extraneural compression. In immobile areas, such as the skull, dynamic neural compression may only be produced by neurovascular mechanisms (Macionis 2023). However, a double crush syndrome involving mechanosensitivity of the trigeminal nerve (refer ‘double crush syndrome and sensitisation’) involving mechanosensitivity of the trigeminal nerve as the C2 DRG is unique in being posterior to the C1-2 facet joint (Haberberger et al 2019), whereby the encasing of the C2 DRG, either within the posterior atlanto-axial ligament, or within an investing fascia that holds the C2 DRG, roots and spinal nerves against the capsule of the C1-2 facet joint (Bogduk 1981) permits substantial mobility of a mechanosensitive C2 DRG.
However, it should be remembered that even in immobile areas sensory nerves can still be mobile because of the dynamic structures they traverse or terminate in e.g. the trigeminal branches glide because of facial muscle activity. Therefore, even in these cases both para- and intra-neural fibrosis can still result in intermittent nerve compression (Macionis 2023).
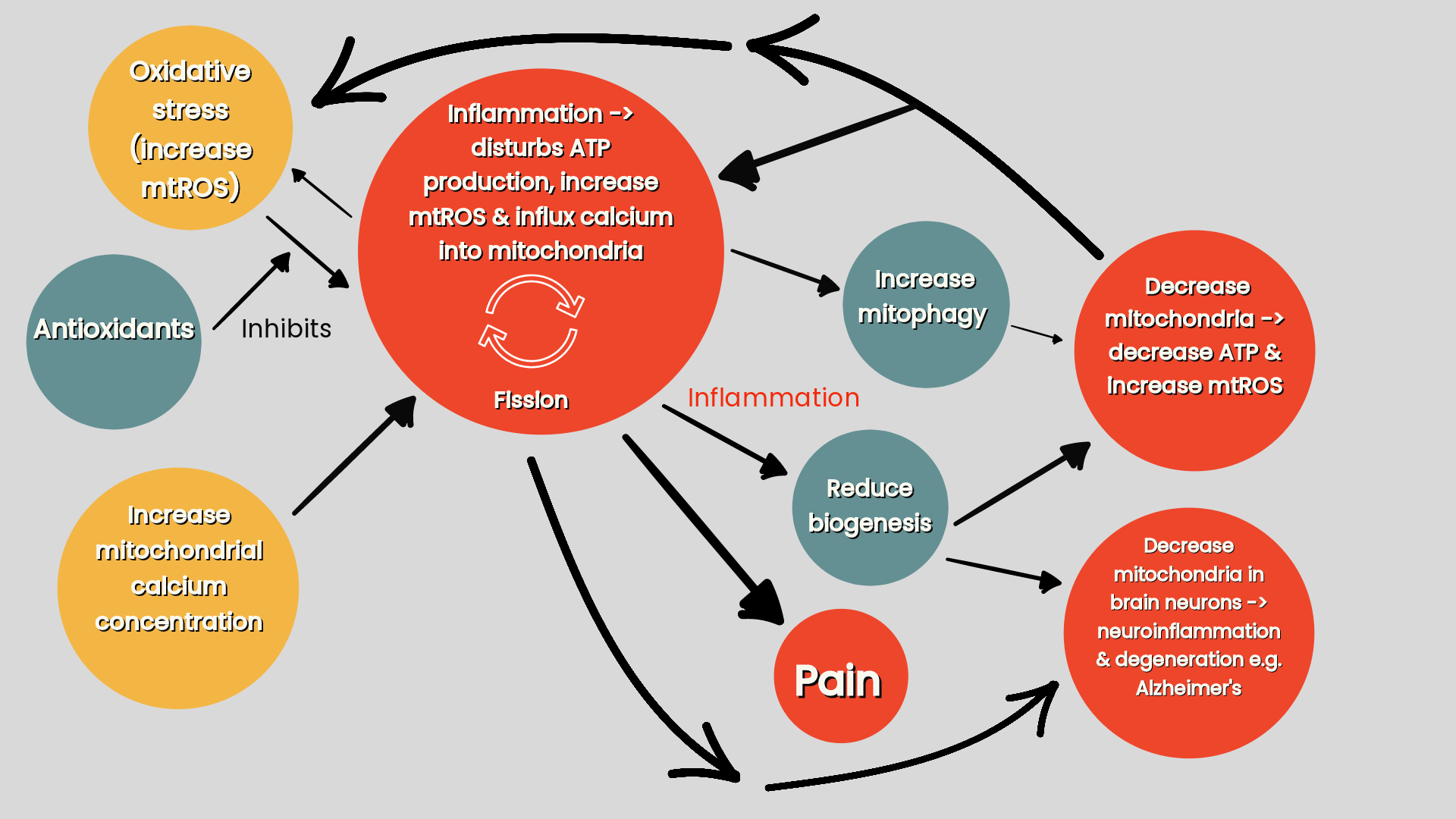
Figure one: Chronic pain cycle. (Macionis V. 2023. Chronic pain and local pain in usually painless conditions including neuroma may be due to compressive proximal neural lesion).
Double crush syndrome and sensitisation
Double crush syndrome (Macionis 2023):
(i). Primary compression —> physically impairs intra-axonal circulation and neuroinflammation —> neural oedema —> impair axoplasmic transport —> activates the DRGn (Macionis 2023). Impairing axoplasmic flow reduces the axonal transport of mitochondria (refer ‘figure three’) impairing nerve structure and function, promoting inflammation, pain, excitability, demyelination and effects the release of neurotransmittors (Silva et al 2022).
(ii). Secondary lesions. Secondary lesions do not necessarily have to be compressive but may entail genetic mechanisms or systemic neuroinflammation producing neural oedema and impairing axoplasmic transport (Macionis 2023).
Because the site of primary compression impairs axoplasmic transport and causes swelling in the nerve, this makes it more prone to secondary nerve compression and causes axonal mechanosensitivity along the whole course of the nerve. Therefore a secondary lesion to this already swollen and sensitised nerve not only disproportionately activates the DRGn to a sum greater than the insult of each lesion individually but makes the nerve trunk more susceptible to further damage and produces disproportionate axonal degeneration and neurological deficits (Macionis 2023).
Just as a disruption of axoplasmic transport facilitates secondary nerve compression and causes axonal mechanosensitivity, neuroinflammation induces both axonal mechanosensitivity and ongoing nociceptor activity. However, in reality neuroinflammation is likely to accompany all nerve injuries (Macionis 2023).
Distal vs. proximal neural lesions
Distal peripheral nerve lesions lead to proximal neural involvement in the form of DRGn ectopic hyperexcitability, DRG fibrosis and neuronal death in the dorsal horn. The more proximal the injury the more detrimental this process is (Macionis 2023).
Increases in axoplasmic calcium following axonal damage triggers Wallerian degeneration. Retrograde signals sent from the injury site to the DRG cell bodies increases production of the growth and survival factors that aid neuronal regeneration. But as well as initiating the production and transportation of trophic functions to aid neuronal regeneration these signals also contribute to inflammatory changes. This results in DRG neuronal cell death and nociceptive changes producing hyperactive, spontaneous ectopic impulses in the surviving DRG neurones and eventually spinal cord central sensitisation (Martin et al 2019). As Wallerian degeneration not only occurs locally, but also distally from the injury site, potentially extending over the length of a nerve, a level of greater sensitisation occurs not only from its ‘spreading’ deymyelination effect but also by inducing spontaneous activity in the neighbouring uninjured C-fibers in the nervi nervorum (Macionis 2023). For instance, lesions to the L5 ventral (motor) root fibers produce inflammatory products, that spill over on to, and in turn produce spontaneous activity in adjacent uninjured L4 nocicpetive C-fibers (and L5 nociceptive afferents) producing mechanosensitivity in the form of mechanical hyperalgesia (Wu et al 2002). This sensitisation is heightened further in the case of proximal, rather than distal nerve lesions (Macionis 2023).
Compressive neuropathic aetiology of generalised chronic pain: the vicious cycle
Chronic pain may be caused by compensatory overuse-related compressive proximal neural lesions (Macionis 2023).
Initial posttraumatic pain —> postural protection —> compensatory musculoskeletal weakness and overuse (Macionis 2023).
Weakened muscles are naturally more susceptible to overuse and myofascial tension which can result in proximal nerve compression (Macionis 2023). This can be seen in migraine patients where decreased strength of the longus colli muscle leads to diminished neck flexion strength and altered head positioning predisposing to sensitisation of nerves in the upper cervical spine (Del-Blanco-Muñiz et al 2023).
It is damage to the motor fibers that mediate the reciprocity between loss of muscle strength and compressive proximal neural lesions. Wrong posture, ligament laxity and muscle shortening may also contribute to development of compressive proximal neural lesions as seen, for example, in the biomechanical pathogenesis of TOS (Macionis 2023).
Primary CNS lesions that activate antidromic activation of the DRGn (action potential traveling in the opposite physiological direction), peripheral tissue damage or radiculopathy —> hyperexcitability of DRG neurons —> proximal reflexive muscle spasm and myofascial tightness, which, due to its compression at anatomically narrow spaces (compressive proximal neural lesions) —> production (or aggravation) of focal neuroinflammation of the proximal nerve trunk at the compression site. This compression maintains this cycle by inducing hyperexcitability of the DRG neurons (Macionis 2023).
It is essential however that the nerve pain be of a persistent proximal neural lesion of intermittent character. Proximal for its potent effects on the CNS and intermittent so that it doesn’t kill off the nocicepetive fibers (Macionis 2023).
This cycle may be perpetuated by: (i) reciprocity between compressive proximal neural lesion caused by motor fiber dysfunction and muscle weakness leading to muscle imbalance and compensatory overuse; (ii) sensitisation as sensitised nerve trunks become more susceptible to further damage. Therefore, a single-site compressive proximal neural lesion, by sensitising the primary nerve trunk, then the secondary nerve trunks that are associated with the primary nerve trunks DRG, and finally, through sensitisation of the CNS, a sensitisation of peripheral nerves all over the body, may result in multiple-sites of compressive proximal neural lesions. This may be another explanation of bilateral widespread chronic pain (Macionis 2023).
Why does occurrence of chronic pain vary from patient to patient?
Susceptibility to pain may represent a predisposition to compressive proximal neural lesions from (Macionis 2023):
Age: gradual decline of muscle strength.
Women: relatively high susceptibility of their musculoskeletal system to overload and injury leads to consequential compressive proximal neural lesions.
Genetics. For example, the growth and extracellular matrix architecture and paraneural anatomical peculiarities (e.g. the dimensions of the anatomical tunnels the nerves travel through) (Wiberg et al 2019).
Neurophysiology.
Explanation of trigeminal neuralgia, temporomandibular pain, and other orofacial pain
Because of relative shortness of the trigeminal nerve, its lesion at any level can be regarded as a peripheral nerve lesion. In trigeminal neuralgia, the trigeminal nerve is compressed (most often via a neurovascular mechanism) proximal to its ganglion, while in TMD the more mobile V3 part of the nerve is affected (Macionis 2023).
This creates a predisposition for motion-maintained intermittent compressive proximal neural lesions of V3 at:
Its exit from the skull (foramen ovale, greater wing of sphenoid) which is very close to the trigeminal ganglion (Macionis 2023).
Superior head of lateral pterygoid: middle deep temporal nerve passes through this muscle (Kwak et al 2003).
Fusion between the deep temporal and lateral pterygoid fascia: buccal nerve (V3) and anterior deep temporal branches of trigeminal nerve (V3). The buccal nerve (V3) after passing within the fused lateral pterygoid and deep temporalis fascia lies against, or imbeds within, the most medial fibers of the deep part of the temporalis (Gaughran 1957). The anterior deep temporal branches of trigeminal nerve (V3) run in the temporalis muscle.
Interpterygoid fascia: the auriculotemoporal nerve (V3) pierces the interpterygoid fascia (Barker and Davies 1972).
Lesions of a single peripheral division of the trigeminus (e.g. V3) inducing inflammation in the trigeminal ganglion (Macionis 2023), and, excitation due to the inflammatory spill over from Wallerian degeneration on to uninjured V1 and V2 branches, leads to sensitisation and a spreading of symptomolgy into the V1 and V2 nerves (Wu et al 2002).
Explanation of low back pain
Thoracolumbar fascia changes in chronic pain
A blockage of normal movement in back pain patients, as opposed to those of normal controls, creates a thickening of the thoracolumbar fascia, as well as a reduced difference in thickness in its longitudinal and transverse axes. Normally the longitudinal axis of the thoracolumbar fascia should be more rigid, as it works as a tendon connecting different body segments and different muscles, and the transverse axis of the thoracolumbar fascia should be more adaptable. This allows the thoracolumbar fascia to have a good adaptive capacity that is different in multiple directions of movement. However when the longitudinal and transverse axis of the thoracolumbar fascia become thickened, denser and more fibrotic it loses its anisotropic behaviour creating what the authors described as a “frozen back” (Pirri et al 2023).
Altered movement patterns creating repetitive stresses remodels the thoracolumbar fascia over time so that this repeated microinjury and inflammation influences nociceptor activation. The resultant mechanosensivity of nociceptive nerves when the thoracolumbar fascia is strained also alters body movement patterns and maladaptive tissue remodelling (Pirri et al 2023). Adhesions that develop between thoracolumbar fascia layers and epimysium of the erector spinae and multifidus alters the thoracolumbar fascia’s function in transmitting myofascial forces which modifies proprioceptive input either directly from the thoracolumbar fascia (Pirri et al 2023), or, from the inside of the muscle out to the thoracolumbar fascia (Brandl et al 2022 & Fede et al 2021).
Facsial-intramuscular anatomy
The aponeurotic fascia, e.g. the thoracolumbar fascia, is continuous through myofascial expansions to the epimysium that covers the outside of the muscle to merges into the paratenon and acts as superficial tendon or aponeurosis that inserts into connective tissue of the locomotor system or into adjacent muscle. The epimysium merges seamlessly with the perimysium that divides the muscle into groups muscle fibers (fascicles). These fasciciles run the length of the muscle to form highly folded interdigitating musculotendinous joints with the tendons and gives attachment for the muscle fibers that do not end in the tendon. The perimysium attaches to the endomysium that covers each individual muscle fiber with the sarcolemma that covers each muscle fiber being attached to the endomysium. The muscle fibers include the regular muscle fibers that lies outside of the fusiform capsule and runs from tendon to tendon (extrafusal muscle fibers), and smaller muscle fibers that are interspersed amongst these extrafusal fibers and are enclosed inside the fusiform capsule (intrafusal muscle fibers).
The intrafusal muscle fibers that form the contractile part of the muscle spindle are mainly bedded within the perimysium (Omstead et al 2023). The muscle spindle’s (fusiform) capsule is continuous with the endomysium, perimysium, and epimysium and fascial septae (Stecco et al 2014), as well as with the ECM of extrafusal fibers and with the perineurium (Stecco et al 2023) and blood vessels (Fan et al 2021). But some intrafusal fibers don’t just terminate within the fusiform capsule they extend beyond it attaching to the intramuscular connective tissue of the extrafusal fibers. This makes the IMCT that the muscle spindle is embedded in a tensile scaffolding that maintains muscle fiber integrity whilst transmitting distal tensions between different muscles, various spindles within the same muscle, and various spindles in different muscles. Being embedded in this network wide tensile scaffolding allows an individual muscle spindle to not only function as an isolated mechanoreceptor, but to pick up, sense and adapt to more distal tensions within the diffuse fascial network within it is embedded (Fan et al 2021). Just as lengthening of a distal muscles preloads muscle spindles through their distal myofascial connections lowering their threshold for firing (Smilde et al 2016) contraction of a synergistic muscles unloads the agonistic muscle spindles to reduce their firing rate (Maas et al 2021). Therefore through these attachments elasticity of the aponeurotic-epi-peri-endomysium–fascial complex is a critical factor in maintaining proper muscle spindle function (Brandl et al 2022) to coordinate the synchronous timing and intensity of each muscle segment’s activation in relation to the intended movement direction (Stecco et al 2023).
Figure two: continuation between the aponeurotic fascia (thoracolumbar fascia or gluteal fascia) —> epimysium covering the outer surface of the muscle —> perimysium covering the fasciculus (group of muscle fibers) —> endomysium (covering each muscle fiber).
Golgi tendon organs and muscle spindles
Golgi tendon organs (GTO) feel the state of muscular force and contraction of the various muscular bundles as they are located, not in the thoracolumbar fascia, but in the gluteal fascia and the perimysium, endomysium and the intrafusal muscle fibers of the muscle spindles (Fede et al 2021). Coordinating force feedback from the GTO (Ia) and length feedback from muscle spindles (IIb) allows an estimate of the length of the muscle-tendon unit to be derived which could be especially important when movement of the muscle fascicles/connective tissue and muscle-tendon complex fail to correlate such as during postural control and locomotion (Nichols 2017). Another feature is that changes in length or position of one muscle, through direct (e.g. hamstring) or fascial (gluteus maximus-fascia lata) connections, affects loading of a distal musculotendonous junction through its anatomical connections (e.g. gastroc-achilles) so that when preloaded its GTOs fire in response to a lower force, and when unloaded GTO’s require greater force to fire (Maas et al 2022). By comparing signals from more distal GTOs (e.g. gluteus maximus and hamstrings) with more proximal GTOs (gastroc-achilles) the proximal–distal force transmitted through the fascia can be estimated (Nichols 2017).
Repeated encoding of these proximal-distal force transmissions through the muscle spindes and GTO’s builds a database so that prior to movement there is a template for what the proximal and distal spatial distribution of tension and movement is expected to be. This template details the preparatory goal-directed gamma motor tuning of the agonistic intrafusal fibers, and also inhibition of the anatgontic intrafusal fibers, to pre-program muscle spindle’s proprioception prior to movement. Teeing up the intentional degrees of spindle sensitivity in anticipation of a planned movement allows gamma motor neurones to exert a modulated reflex control over the appropriate levels of muscle stiffness for initiation and execution of a specific movement and postural control (Torell et al 2023). Unlike beta motor neurones gamma neurones are unaffected by movement as their control over alpha motor neurones and muscle spindle activity is one way ensuring they can function independently from both. Being autonomous from alpha motor neurones ensures gamma motor neurones are not swayed by either any coinciding alpha-generated muscle force (Torell et al 2023), or, from muscle spindles that may otherwise cause gamma motor neurones to short circuit themselves through their own actions (Khan et al 2022). But whilst gamma motor neurones function independently from alpha motor neurones and muscle spindles they are under control from top-down commands, peripheral inputs informing of bodily states from (i) visual input: as seen by how babies observe and mimic to develop proprioceptive control; (ii) joint receptors facilitate nonspecific spinal pathways to initiate joint protective reflexes (Sjölander & Johansson 1995); and (iii) cutaneous receptors tactile stimulation from kinesiology tape improves proprioception through gamma motor neuron firing, Lin et al 2021; (iv) proximal-distal mechanical tensions. The integration of all these different sources of information by gamma motor neurones converges this sensorimotor information on to the spindles to create a flexible representation of posture and limb kinematics (Dimitriou 2022), a template of “gamma-planned movement”. When this pre-planned movement is eventually executed coordinated feedback is received from (i) the GTOs on the directional patterns of stress, and (ii) the changes in length as a result of this stress (Nichols 2017) by how beta motor neurones prevent the spindles from falling slack in order to maintain their sensitivity (Khan et al 2022). However, beta (and intrafusal muscle) activity can be overactivity if prolonged gamma motor activity stimulates muscle II, III and IV chemo and nocieptive afferents (Partanen et al 2023) (refer figure ***). Therefore it is preparatory loading of the muscle spindles by the gamma motor neurones allows the nervous system to selectively extract information that compares what the intended spindle signal would be as a consequence of the movement with what they actual were. Any deviations from this template either updates this template and permits this novel movement, or alternatively, the template having identified flaws reflexly corrects them to bring the movement back into its pre-programmed stereotypic pattern. This provide a mechanism for proprioception and kinaesthesia (Nichols 2017).
However, when instead of a fine gamma motor neuron firing to execute a nuanced muscle stiffness in preparation for a dynamic movement, a full on, static, isometric contraction is required gamma motor firing is wholesale. In such a case gamma generated intrafusal muscle contractions in the agonistic muscle (with contributions from the beta motor neuron), is combined with the inhibition of intrafusal muscle activity from the antagonistic muscle, to reflexly produce an alpha-generated extrafusal muscle force (alpha-gamma co-activation) in the form of an isometric contraction (Dimitriou 2022). If, immediately after this isometric contraction, the muscle is passively shortened the muscle spindle then slackens and de-activates the alpha motor neuron.
Figure three. Extrafusal muscle fibers are located outside of the fusiform capsule (blue line) innervated by the alpha motor neurones and directly induce muscle tissue contraction or relaxation.
Intrafusal muscle fibers: are located inside the fusiform capulse (blue line) being interspersed between the extrafusal fibers. Central portion is not contractile being innervated by myelinated Ia or primary afferents that coils around it to form the annulospiral ring (black circular line) which provides information about the length and velocity of the muscle whilst it is moving, but as soon as the muscle stops lengthening Ia rapidly adapts to new length and stops firing. Stretching the muscle relative to the muscle spindle —> stretches the annulospiral ring —> stretch reflex = alpha motor activity activated to extrafusal fibers, but inhibit alpha motor activity to the antagonistic muscles extrafusal fibers. Relaxing the muscle spindle allowing it to shorten deactivates this stretch reflex = muscle relaxation. Peripheral portion is contractile being innervated by gamma motor neurones and medium myelinated II afferent fibers that both terminate with flower-spray endings. These only provide information on muscle length, but keep firing even when the muscle is at rest. Being slow to adapt to the new length II keeps firing reporting on the new body position even once it has stopped moving. Gamma motor neurone activation —> contract intrafusal sarcomeres in the periphery of the intrafusal muscle fiber —> pulls tight the central portion —> stretch reflex (=muscle contraction). Conversely, gamma motor neurone deactivation relaxes (shortens) the central portion of the intrafusal fiber resulting in muscle relaxation. These sensory nerves carry information to the CNS about the magnitude and speed of muscle stretch and proprioception.
As alpha motor neurones cause extrafusal muscle fibers to contract, the intrafusal muscle fibers also contract (‘4’) controlling the length of the intrafusal muscle fiber as not to result in ‘3’. By maintaining the sensitivity of the muscle spindle to stretch allows it to provides fine compensations of muscle length and velocity. During movement beta motor neurones stop the muscle spindle falling slack modulating its sensitivity as to reflexly modulate activity of the alpha motor neurones and subsequent muscle contraction, and during isometric contraction gamma motor neurones keep the muscle spindle tight to the same end.
Aponeurotic fascia (e.g. gluteal or thoracolumbar fascia) adhesions and reduced epi-peri-endoneurium elasticity pulls the muscle spindle tight, preventing it form relaxing (shortening), leading to sustained activation of the stretch reflex (=muscle contraction) and failing to smooth out neural irregularities (=spasmodic contractions).
Fascial-intramsucular force transmittion
More than 30% of the mechanical forces are transmitted from the aponeurotic fascia through its myofascial expansions to the epimysium (Stecco et al 2023). Adhesions between the thoracolumbar fascia and epimysium of the erector spinae and multifidus (Brandl et al 2022) and the contractile properties of the myofibroblasts in the aponeurotic fascia and perimysium (Schleip et al 2019) create a direct line of pull. This tension through the continuous intramuscular fascial chain from the epimysium to the muscle spindles stimulates GTOs along its course (Fede et al 2021) and stretches the muscle spindles.
As most of the intrafusal fibers that make up the muscle spindle lie within the perimysium (Omstead et al 2023), and the fusiform capsule the intrafusal muscles lie within is connected to the epimysium and fascial septae (Stecco et al 2014), tightness in this connective tissue transmits any pull from the myofibroblasts, aponeurotic fascia-spimysium adhesions (Brandl et al 2022) or distally from the adjoining myofascia (Maas et al 2022) directly to the spindles. Preventing the muscle spindles from shortening and switching off this tightness and adhesions keeps the spindles stretch out and stimulated during muscle contractions with ensuing (i) stretch reflexes resulting in muscle contraction; (ii) inhibition of the muscle spindles function in addressing irregularities that would otherwise allow a smooth muscle contraction resulting in multiple spasmodic twitches (Brandl et al 2022); (iii) sustained intrafusal muscle firing that stimulates Ia fibers results in mechanosensitivity (Johnson et al 2019) and fatigue and inflammatory metabolites that activates both small diameter (type III & IV) nociceptive fibers (Partanen et al 2009) and large diameter Ia and II fibers. This sustained intrafusal muscle firing also releases glutamine, which, as their primary source of fuel is taken up by macrophages in the muscle spindle’s fusiform capsule to convert this glutamine into GLU. The macrophages then release this GLU to activate the Ia afferents GLU receptors (Yan et al 2025). Wether through mechanical pull, fatigue and inflammatory metabolites or release of GLU sustained intrafusal muscle firing stimulates Ia and II fibers to activate astrocytes and cause Ia and II ectopic and anti-/orthodromic firing that increases both mechanosensitivity and stimulate nociceptors causing peripheral and central sensitisation (Sas et al 2024). The highest density of paraspinal muscle spindles are from the lower thoracic spine to L3-L5, the overstimulation of which results in the deformation and increased stiffness of the thoracolumbar fascia in low back pain patients (Brandl et al 2022) (refer ‘figure three’).
Conversely, the intramuscular connective tissue not only transmits forces from outside (epimysial fascia) —> in (muscle spindle) but also from inside (muscle spindle) —> out (epimysial fascia). Activation of the intrafusal fibers will not only create tension in the fusiform capsule to which it attaches (and in turn the intramuscular connective tissue to which the capsule is attached), but for the intrafusal fibers that pass beyond the capsule contraction will tighten the intramuscular connective tissue of the extrafusal fibers. The endomysium is directly in contact with the sarcolemma that surrounds each muscle fiber so that force generated by a muscle fiber is transmitted directly to the endomysium. The endomysium is one continuous fascial network, although it is partially made of fibers that spiral individual muscle fibers it is mainly made of individual fibers that run longitudinally to prevent over-elongation and -contraction (Järvinen et al 2002) and also individual fibers that envelope multiple muscle fibers within the fascicle (Stecco et al 2023), and attach the capillaries and nerves to the surrounding muscle fibers (Järvinen et al 2002). Collagen fibres of the endomysium elongate from a circumferentially to longitudinal orientation when the muscle is stretched. Being compliant in tension along the muscle fibre direction means the endomysium doesn’t restrict changes in muscle fibre length and diameter as muscles contract and relax, but, conversely being so compliant in this direction means that is also inefficient in transmitting forces longitudinally. However, the endomysium is resistant if traction is lateralised by the active contraction of muscle fibers that are lateral to non-activated muscle fibers. Therefore laterally activated muscle fibers pulling its endomysium tight means a uniform strain can be maintained over the endomysium of more medially non-activated fibers. This allows these non-recruited fibers to become a tendon for the transmission of lateral force without having to change the length. The endomysium passes uninterrupted to the perimysium whose perpendicular fibers allow lateral binding of the muscle fibers and connections between synergic muscle fibers of different fascicles, whilst permitting independence between muscle fascicles during muscle contraction. These intramuscular connections, along with its strong resistance to traction means that, unlike the epimysium, instead of contributing to passive rigidity it transmits intramusclular forces outwards towards the bone (Stecco et al 2023). When intrafusal muscle fibres, that make up the muscle spindle, transmit forces out diffusely through the continuous fascia network of the endoneurium, and from there to the peri-epimysium, the nerve networks in the epimysium and aponeurotic fascia coordinates and unifies all this tension emanating from the muscle spindles, that represent proprioceptive and tonicity states, before forwarding it to the central nervous system (Fede et al 2021) (refer ‘figure four’).
Figure four: from outside-in the muscle spindles can be pulled tight (refer figure four) from aponeurotic fascia (e.g. thoracolumbar or gluteal fascia)-epimysium adhesions or contractile force from the myofibroblasts in the aponeurotic fascia or perimysium as most of the muscle fibers are located in the perimysium. Conversely, the muscle spindle can itself exert traction from inside-out to the aponeurotic fascia.
This traction effect activates afferent nerve endings at multiple levels including the golgi tendon organs. Sustained intrafusal muscle firing releases GLU from Ia afferents (annulospiral ring), neuropeptides from nocicepetors (=neurogenic inflammation) and pain and fatigue metabolites —> mechanosensitivity, DRG & central sensitisation & reflex gamma activation —> intrafusal muscle firing in a positive feedback loop. Each gamma axon innervates several spindles in the same and neighbouring muscles.
Figure five: (1) sustained intrafusal muscle firing, and extra- and intra-muscular adhesions, myofibroblast contracture and increase HA concentrations through biochemical and biomechanical causes stimulates muscle spindles. (2a) stimulating muscle spindles increases Ia and II firing that releases GLU from nerve terminals. (2b) intrafusal muscle contraction release glutamine, muscle spindle macrophages (x) convert glutamine to GLU —> GLU activates Ia afferents GLU receptors. Orthodromic release of GLU = stimulation of nociceptors and peripheral sensitisation and antidromic release of GLU = central sensitisation.
Muscle-fascial stiffness - defining a muscles passive resistance to stretch
Titin
The sarcomere contains two interdigitating myosin thick filaments and actin thin filaments that slide by overlapping each other during contraction, reducing this overlap during relaxation and separate the actin-myosin filaments so the overlap is absent when the muscle is stretched. As well as myosin cross-bridges sliding actin towards the midline of the sarcomere during contraction, and away from it on relaxation they probably rotate actin as well (Monroy et al 2012). But such a simple model would leave the sarcomere unstable, disorganised and misaligned during passive stretching with this instability further exacerbated when, in the stretch position, force is then produced such as during eccentric contraction or an isometric contraction when the muscle is under stretch. Stability can be obtained with a third elastic sarcomeric filament titin. Each titin filamentous protein spans half the sarcomere being anchored to (i) the boundary of the sarcomere (Z-disk). At the Z-disk titin attaches to the periphery of the disk and to actin from the same and adjacent sarcomeres. As it moves away from the Z-disc (into the I-band) towards the midline titin and actin filaments separate. (ii) The distal part of titin’s I-band attaches to the tip of the myosin filaments and then through its A-band it attaches onto the myosin filament all the way through to a central vertical line (M-line) where the myosin filament from one half of the sarcomere links to the other (Linke 2023). Establishing a permanent bridge between actin and myosin the overlap of titin molecules at both ends (Z-disks and M-lines) produces a titin filament system that is continuous along the entire length of a muscle fiber.
The peripheral (I-band) region of titin is composed of spring elements that lengthens when the sarcomere is stretched giving rise to a passive force that creates fluctuating tension in the form of elasticity from its proximal attachment to the Z-disc to its distal attachment at the tip of the myosin filament. In contrast through its myosin attachments its centre (A-band) region is stiffer and inextensible as it functions to stabilise myosin (Noureddine & Gehmlich 2023). Myosin needs to be stabilised by titin as during contraction when one half of the sarcomere’s myosin motors are switched on it becomes stronger and shortens pulling myosin from it central position. Conversely in the other relaxed half of the sarcomere the myosin motors are switched off reducing the force from the lack of cross-bridges so it gets stretched over towards the stronger contracting side as it shortens. This stretching of the weaker ‘switched off’ half-sarcomere is resisted by titin that elongates to not only keep myosin in the centre of the sarcomere but also switches ‘on’ the myosin motors. Therefore titin, along with the effects of stretching in reducing the space between actin and myosin filaments and increasing actin’s Ca2+ sensitivity primes the cross-bridges (Linke 2023). Priming the cross-bridges so they are only weakly bound means that on Ca2+-actin activation titin can bind to actin so it stretches and strengthens the cross-bridges allowing them to metabolise ATP and begin the cross-bridge cycle. Through this process the weaker-stretched half sarcomere can transition to the stronger-contracting half sarcomere. In this way by equilibrating forces in the two halves of the sarcomere titin helps maintain force and velocity on muscle shortening (Squarci et al 2021).
When jumping we naturally make a counter-movement (eccentric contraction or active stretch) by squatting down before the main movement (concentric or shortening phase) of jumping up. By not using ATP and being elastic titin has a low energetic cost as it generates pent up energy in the counter-movement phase by lengthening sarcomeres under tension (= generates stiffness) and then rapidly releases this energy during the main movement that shortens the sarcomeres under a low load and high velocity. Therefore during this stretch-shortening cycle titin’s viscoelastic properties can be utilised by undergoing the following three phases: (i) stretch; (ii) lengthening contraction; (iii) active shortening or relaxation.
1. Passive stretch. When stretching the sarcomere beyond its normal physiological range (beyond the actin-myosin overlap) the peripheral I-band region of titin unbunches from itself, and actin, to straighten out and stretch. Firstly the proximal Ig region is stretched and then, as the more elastic PEVK region is stretched at longer sarcomere lengths, muscle stiffness increases steeply. Stretching improves actin’s Ca2+ sensitivity that helps bind titin to actin, which, along with Ca2+ also binding to titin, serves to increase titin’s stiffness disproportionately to the stretched sarcomere’s length. When the myosin motors are turned off and cross-bridges are reduced this allows tension to be transmitted from titin’s proximal Z-disk attachment (I-bands) to its distal myosin attachments (myosin tip, A-band and M-line). This allows when stretched titin to improve cross-bridge interactions to allow more force to be generated by less Ca2+ (i.e. reduced frequency of action potentials). This is through titin’s shared interactions at the Z-disk with actin creating a stress induced distribution of charges within actin that increases actin’s affinity for Ca2+ (Joumaa & Herzog 2014) and titin (PEVK) (Squarci et al 2024), and (ii) by drawing actin and myosin closer together (Joumaa & Herzog 2014).
2. Lengthening contraction (eccentric loading or active stretch). Once titin is elongated with a passive stretch muscle activation causes titin to bind to actin elongating it even further. Passively stretching titin to increase its Ca2+ sensitivity means that during active contraction, when action potentials eventually release Ca2+, titin can bind to actin stretching it more so than it was during the passive stretch. This active stretch of I-band titin transmits tension to its myosin attachments to turn on and prime the myosin motors by making the preliminary steps to myosin activation (Squarci et al 2023). As power ramps up in these primed cross-bridges the interaction of myosin with the distal Ig segment and part of the PEVK region causes it to get mechanically entangled with the rotating cross-bridges. This causes titin to shortens and move towards the M-line which further lengthens and stretches the PEVK and proximal Ig region as it unfolds (DuVall et al 2017) ramping up power myosin motors. Titin elongates in this lengthening-contracting phase by binding to actin in two phases.
Phase one: titin’s N2A region binds to actin. When Ca2+ is released during active contraction titin’s N2A region binds to actin. This allows Ca2+ to have a direct, but relatively small effect on further increasing titin stiffness. But this binding of titin to actin is velocity dependent as higher velocity stretches detaches titin from actin so titin fulls more slack compromising the subsequent priming of cross-bridges in the lengthening-contraction phase. However, slower velocity stretches will also eventually lead to detachment of titin from actin if progressed causing its priming effects to level off (Hessel et al 2022). Once the Ca2+ release from active stretch or eccentric contraction binds the N2A region of titin to actin it means the Ig regions can’t straightening out as to reduce titin’s free length and increases its stiffness. This means that any further elongation in this lengthening contraction phase has to come from the stiffer, more elastic, PEVK region (Monroy et al 2012).
Phase two: upon muscle activation, either physiological-resting activation, or, as discussed here, during the lengthening contraction phase, titin’s PEVK segment coils around the much stiffer actin filament. This coiling of the PEVK segment around actin on activation increases the effective I-band titin stiffness regardless whether the muscle is activated on stretch or normal physiological length (Squarci et al 2023). This PEVK-actin binding is due to Ca2+ sensitivity, but, primarily is due to electrostatics. The - charged poly-E regions interact with the + charged regions on actin, and the + charged PPAK motifs interact with the - charged regions of actin. As the poly-E region (-) and PPAK motifs exhibit opposite charges they experience repulsions between each other resulting in electrostatic stiffening.
However, in prolonged active muscle -> CO2 -> lowered pH -> poly-E regions GLU (-) has protons (H+) added to it so GLU converts to GLU+ so the poly-E region now becomes neutral charged. The reduced attraction between (i) the now + charged poly-E region and + PPAK region, and (ii) the now + charged GLU+ within the poly-E region means this area start to give and uncoil to increase poly-E flexibility. This overall softening of the PEVK allows for it, upon stretch, to be pulled a part so mechanical energy can be stored in the PEVK region (<stiffer poly-E region) like a spring that on recoil releases this pent up energy to aid sarcomere shortening (Sudarshi et al 2020). Ca2+ bind to GLU (-) to decrease its charge repulsion so that subregions of poly-E bunch up resulting in increased PEVK stiffness. PPAK motifs interact with GLU- on the poly-E motif and so compete for shared binding interactions between Ca2+ and GLU- sites with the resultant blocking of poly-E Ca2+. Whilst passive stretching pulls apart PPAK motifs and poly-E motif to reduce their interaction and increase stiffness the influx of Ca2+ during contraction maintain flexibility of the poly-E region, reducing PEVK stiffness and allowing longer sarcomere lengths to be achieved more easily. to the changes in Na+ concentration and that this might represent an additional mechanism for tuning PEVK stiffness.
During lengthening contraction actin is drawn off of myosin and passively rotates in a counter-clockwise direction (as seen from the Z-disk). At the beginning of an isometric contraction the actin-myosin detachment reduces cross-bridge resistance so the “chain slips off” enabling actin to lengthen out by passively rotating counter-clockwise on the spot (with no sarcomere shortening) for 5-8 secs. Eventually this winding up of stress in actin produces torque and tension as it elongates it attachment to the Z-disk by first unwinding and then winding back up its attachment fibers, which along with the eventual re-formation of cross-bridges, causes this counter-clockwise rotation of actin to cease (Jarosch 2008). As titin is attached to the rotating actin, and titin is attached to both myosin and actin, every cross-bridge interaction twists titin around actin to (i) stop actin from unwinding, and (ii) create a titin-spring of elastic energy around actin that as it uncoils during active shortening or passive relaxation allows this energy to be recovered (Monroy et al 2012). So the peripheral I-band region of titin is lengthened during passive stretch as the sarcomere lengthens and then again when isometric contraction in this lengthened contraction state coils titin around actin to half its length and twofold increase in tension (Squarci et al 2023). But the the cross-bridges forces winding titin on actin to build up elastic energy in its peripheral region can’t increase indefinitely it has to be balanced and countered by equal force at titin’s attachment to the Z-disk. Sarcomeres of fast-type muscles, e.g. psoas, are typically more stiff as the titin (I-band) that twists around actin is shorter storing more potential energy in its tightly coiled spring, whereas sarcomeres of slow-type muscles, e.g. soleus, that are typically three times less stiff, have a longer titin (I-band) that stores less potential energy as it coils around the actin (Linke 2023). But as titin is viscoelastic its stored elastic energy in the lengthened contraction phase not only increases with increasing degrees of stretch but also stretching velocity (Tomalka et al 2021).
If a muscle is allowed to relax during this lengthening-contraction phase within one second cross-bridges detach (Fukutani et al 2021) and some of titin’s elastic energy generated in the lengthened contraction phase decays. But with the muscle being still being in a lengthened position, all be it a passively lengthened position, some of titin’s stiffness will still persist for several seconds after being returned to a neutral position resulting in a ‘passive force enhancement’ that increases efficiency of subsequent muscle contractions.
3. Low load rapid active shortening or shortening-relaxation. This passive and active stretching of titin by lengthening the sarcomere and binding and coiling titin around actin, (i) transmits tension through titin to its myosin attachments (distal Ig and A-band) to turn on the myosin motors and form the cross-bridges needed for active shortening (concentric contraction) (Squarci et al 2023); (ii) strengthens the cross-bridge hold that binds the actin-myosin filaments together. As opposed to a static isometric contraction during active contraction, or passive shortening, the strength of the cross-bridge grip has to be released. Releasing the tight grip of the cross-bridges allows actin to slide and clockwise rotate towards the midline (Jarosch 2008) before new cross-bridges are again formed. A cyclic free and easy attachment-dissociation of actin-myosin filaments allowing actin to drill towards the midline is characteristic of a low load-high velocity sarcomere shortening. Therefore, after being wound up and stretched titin has to be rapidly shortened to release its elastic energy and switch off the myosin motors to release cross-bridge force. In this switched off-relaxed state the cross-bridges are incapable of impeding the rapid shortening of the sarcomere that releases titin’s elastic energy necessary for a free and easy shortening of the muscle. The recoil of titin involves detaching titin from actin in two phases:
Phase one: detachment of titin (N2A) from actin. In the lengthening contraction phase titin increases cross-bridge force so more force can be generated by less Ca2+ (i.e. reduced frequency of action potentials). But through its shared interactions at the Z-disk with actin shortening titin decreases stress, and alters the distribution of charges, within actin as to decrease actin’s affinity for Ca2+ (Joumaa & Herzog 2014) and titin (PEVK) (Squarci et al 2024). This, and by physically separating the actin and myosin filaments slackens the actomyosin cross-bridges so less force is required to detach titin from actin and allows for titin’s next phase of energy release.
Phase two: Unwinding of titin (PEVK) from actin. Detaching titin (N2A) from actin only releases a small amounts of titin’s pent up energy, a majority is released from how titin (PEVK) unwinds from actin. As mentioned in phase one cross-bridge forces decline with shortening velocity, or rapid relaxation, so the cross-bridges passively snap from one another dropping the power out of actin and titin that was wound up in the lengthening-contraction phase. Reducing the restraining power of the cross-bridges means nothing can hold titin back from unwinding around actin to release its coiled up energy, and allow for the free and easy clockwise rotation and sliding of actin towards the midline. This further unwinds stress at the shared actin-titin Z-disk attachment to alter the distribution of charges within actin that decreases its affinity for Ca2+ and titin. This is how after five quick releases more prolonged relaxation of the muscle can be achieved (Jarosch 2008). In contrast when slowly lifting a heavy load the acto-myosin cross-bridges that twisted actin and coiled titin around actin in the lengthened contraction phase remain tightly bound in the active shortening phase. The strength of the cross-bridges in keeping the actin and titin wound tight overpowers the energy wound up in the titin spring so the titin-spring stays coiled tight and its energy isn’t released. This restraining power from the tightly bound actomyosin cross-bridges prevents titin from fully unwinding so only a relatively small amount of energy is released restricting sarcomere shortening (Monry et al 2012).
Therefore, titin’s elasticity allows it to act as a tuneable spring regulating muscle stiffness to optimise the individual muscle’s mechanical requirements. These requirements are known through prior experience that details a blueprint of the optimal muscle stretch and contraction for any given movement. This pre-programming tees up the muscle spindles prior to execution as what should occur so that during a movement muscle spindle feedback guides neuromuscular function and the resultant settings of the titin springs for optimal mechanical effect.
Hyaluronic Acid
Hyaluronic Acid (HA) offers mechanical stability, lateral force transmission during muscle contraction, shock absorption and flexibility by acting as a water reservoir that maintains the viscosity and lubrication between two adjacent surfaces. High molecular weight HA helps maintains normal homeostasis by displaying analgesic, anti-inflammatory and immunosuppressive properties whilst neutralising free radicals, whereas low molecular weight HA promotes inflammatory and nociception. Locations requiring high degrees of lubrication and fascial gliding contain more HA per gram of fascial tissue, such as those over synovial joints as seen in the ankle retinacula, whereas areas where the fascia is more adherent to muscle contain less HA per gram of fascial tissue such as in the fascia overlying the trapezius and deltoid (Pratt 2021).
HA is synthesised in response to mechanical stimuli, such as strain and movement, or chemical stimuli including inflammatory mediators and hyperglycemia (Amir et al 2022). It is predominately produced by fibroblasts that when resting (inactivated) produce an ECM, of which HA is a major component, to maintain homeostasis. When activated fibroblasts become highly migratory, proliferative, and increase the production of ECM, including HA. As well as transporting all this HA to the ECM it also remains associated with the fibroblasts cell membrane (via its CD44 receptor), triggering inflammation and differentiation of the fibroblasts into myofibroblasts. Myofibroblasts not only increase stiffness through its contractile properties but also increase production of the ECM including HA. HA is also produced by fasciacytes that are specifically located to permits to modulate fascial gliding and autonomy among the various fibrous sublayers (Stecco er al 2018).
Only 10%-15% of HA is catabolised locally in the tissues, the major part of HA is cleared by the lymph and blood circulation for subsequent degradation in the local lymph nodes and the Liver. Immobilisation leads to elevated ECM content (HA and fibrosis) which increases the interstitial pressure compressing neurovascular structures and increases intrinsic muscle stiffness as it hinders the muscle’s ability to elongate. This could account for morning symptoms whereby stiffness from HA accumulation in the joints and muscles overnight is eased by movement (Amir et al 2022). This reduced compliance along a continuous connective tissue plane in which the muscle spindles are embedded allows force to be transmitted more efficiently to them so they are kept on stretch and prevented from relaxing resulting in muscle spasms by:
(i). Increasing perimysial and endomysial connective tissue which reduces the elasticity and adaptability of the intramuscular connective tissue. Increased collagen fibers deposition in the endomysium is mainly directly around the sarcolemma to form connective tissue septa that simultaneously increases the contacts between individual muscle fibers whilst at the same time separate them from each other. This deposition is most prominent in the perpendicular fibers that connect two adjacent muscle fibers together to restrict the mobility of the individual muscle fibers, and, by also forming around the intramuscular capillaries isolates the capillary, and its blood supply, from the adjacent muscle fiber (Järvinen et al 2002).
(ii) Movement stimulates HA production and turnover, but immobility decreases the normal turnover of HA resulting in its accumulation in an attempt to increase the gliding efficiency between two surfaces. But this accumulation of HA increases interstitial pressure in the deep fascia, between the muscle and the fascia, in the intramuscular connective tissue, the fusiform capsule and the inner and outer fusiform capsular spaces occupied by the muscle spindle and its intrafusal muscle fibers. This increased viscosity and decreased gliding between layers of connective tissue and muscle fibers results in increase its intrinsic stiffness (Stecco et al 2014). Viscosity can be further increased by HA-mediated inflammation that further accumulates HA and when immobility from sustained muscle contraction produces lactic acid lowering the pH to 6.60. HA progressively breaks down with manual therapy and when the temperature is increased to >40 °C with a resulting decrease in viscosity and stiffness (Pratt 2021)
Myofibroblasts
Mechanical strain signals and the anti-inflammatory cytokine TGF-β triggers binding of HA to the CD44 cell membrane receptors forming a HA pericellular coat that is anchored to the fibroblast cell surface. The downstream effect of this HA‐CD44 interaction causes the fibroblast to differentiates into a myofibroblast. These myofibroblasts not only generate a contractile force but increase production of the ECM, including HA, (Tai et al 2021) that increases stiffness in the aponeurotic fascia and perimysium (Schleip et al 2019).
Brief mechanical stretching reduces the levels of TGF-β, but, in contrast, sustained mechanical stimuli increases TGF-β levels, promoting the transformation of fibroblasts into myofibroblasts. This transition into myofibroblasts exacerbates contraction force that in turn activates more fibroblasts to create a positive feedback loop resulting in tissue hypertrophy (especially in cases of acute stertching) and increase contractile force (Li et al 2024)
Treatment techniques
Lengthen-hold-passive relaxation. The aim of this procedure is to slacken the intrafusal muscle fibers and in turn any firing from the muscle spindles. Turning off and slackening the muscle spindles means they are unable to provide appropriate information on length and lengthening of the muscle thereby tricking the muscle spindles into providing sensory information that is not in line with the overall muscle length. This lowers the reflex intensity of the muscle to stretch (Bittmann et al 2023) and decreases the nociceptive responses to muscle spindle firing (Sas et al 2023).
Stretch phase. On more of a macro-level stretching straightens out the muscle fibers so its sarcomere’s align and sit at a shorter resting length so that on shortening the muscle can contract act a greater velocity. On more of a micro-level as the stretch is applied, the cross-bridges are also stretched, resulting in a short period of passive stiffness. Once these cross-bridges are stretched to their limits the force and elasticity in them is overcome as they break and reduce its stiffness. This ‘give’ in the stretch allows the actin-myosin filaments to slide into a lengthened position before new cross-bridges are formed (Blum et al 2020) stiffening back up and fixing the intrafusal fibres at this longer length (Morgan et al 1984). During an active stretch the cross-bridges are more tightly bound by the muscle contraction leading to an increase in force per cross-bridge and the storage of elastic energy that has to be overcome in order for them to ‘give’ and bind back up again in an elongated position (Fukutani et al 2021). This is how eccentric exercises improve flexibility, fascicular length and decrease passive <musculotendinous stiffness (Kamandulis et al 2024). But the actin filaments don’t just detach to slide out to a lengthened position they rotate out in an anti-clockwise elongating and winding up tension in their Z-disk attachments (Jarosch 2008). This lengthening draws actin and mysoin closer together increasing actin’s Ca2+ sensitivity so more attached cross-bridges can form, or transition from weakly to strongly bound cross-bridges (Joumaa & Herzog 2014).
Isometric phase. Strain is induced along the fiber axis and at the cross-bridge when the myosin heads transition from one attached state to the next. As the cross-bridges detach this strain is released and the two filaments slide past each other shortening the sarcomere (Adhikari & Fajer 1996). When stretched so the actin-myosin overlap is reduced the few available cross-bridges that are able to generate force during an isometric contraction will have myosin heads held in a tightly bound, highly strained, inflexible state. Fixing the cross-bridges in this lengthened out position affords no ‘release’ that would otherwise be provided by cross-bridge detachment and filament sliding. This high force generates a positive feedback loop: Ca2+ released by the action potential —> structural changes to actin: allows titin proximal I-band to bind to actin —> increase titin proximal I-band stiffness = increase titin’s distal A-band stiffness that attaches to, and causes structural changes to myosin = stress-dependent activation of myosin motors (Morotti et al 2024) and myosin cross-bridge binding sites —> further increases stress-dependent activation of myosin (Linari et al 2015). But to specifically engage the cross-bridges of intrafusal fibers gamma motor neurones have to be activated by initiating a strong isometric contraction (Gregory et al 1998). Just as this gamma isometric contraction of the intrafusal fibers forms tight cross-bridges that switches the muscle spindle on, a post-isometric relaxation of the intrafusal fibers would detach the elongated cross-bridges to allow further stretch of the intrafusal fibers (Macefield & Knellwolf 2018) but without a further isometric contraction the cross-bridges would not reform.
Passive shortening. When the muscle is at rest, increased mobility of the myosin heads (Adhikari & Fajer 1996) allow for the spontaneous formation and breakage of cross-bridges at a steady predictable rate. Similiarly a freely shortening muscle with minimal cross-bridge force (=low stiffness) allows the actin-myosin filaments to break from each so the filaments can slide (Muthu et al 2008) at a steady predictable rate (Blum et al 2020). However, after being stretched and isometrically contracted the actin filaments and titin, by turning the myosin motors on, ensures the number and strength of the cross-bridges is high (=high stiffness) fixing the actin-myosin filaments in this lengthened position. This means more myosin motors can achieve a super-relaxed state with zero cross-bridge force by using a large magnitude and high velocity shortening movement (Linari et al 2020).
In contrast to the lengthening phase the high velocity relaxed shortening movement causes a clockwise rotation of actin displacing Ca2+ to allow an unwinding of the torque at actin’s attachment to the Z-disc (Jarosch 2008). Switched-on myosin motors initiate movement of the myosin head so actin-myosin can start to move to a shortened position which increases stiffness in the still attached cross-bridges. Further movement then releases the elastic energy stored in the myosin head causing the cross-bridges to come off stretch and slacken (=reduced stress) (Linari et al 2020). Slackening the cross-bridges increases their flexibility allowing them to endure greater deformation (in stretching or contraction) before buckling and detaching (Fenwick et al 2018). This improves shortening velocity as maintaining a greater number of attached cross-bridges affords less reliance on an individual cross-bridge that would otherwise have to ‘grip on’ hard enough to generate enough force and slow cross-bridge cycling velocity (Hanft et al 2015). On further shortening the myosin motors turn off and the cross-bridges detach allowing a rapid sliding of the filaments. The negative pressure produced by movement of the myosin heads when the sarcomere shortens at a low (or suddenly reduced) load and at a high velocity causes premature forcible detachment of the cross-bridges (Linari et al 2020), encourages increased compliance and low stress in the filaments (Fenwick et al 2018), continually decreases the number of cross-bridge binding sites and by decreasing radial compression increases actin-myosin spacing (Hanft et al 2015). The resultant ‘free-sliding’ of the filaments allows them to travel a greater distance before cross-bridges reform (Linari et al 2020), unless, the myosin motors detect even small amounts of stress in which case they re-engage to form new cross-bridges halting the free and easy shortening of the sarcomere (Linari et al 2015). splitting ATP at the myosin head ATP into a ADP⋅Pi complex attaches myosin to actin to turn myosin motors on to form cross-bridges that are primed for movement. Movement of the myosin head during the first phase of contraction releases Pi generating force in and stiffening up the cross-bridge. During the second phase of contraction at low, or suddenly reduced loads, the force and stiffness at the cross-bridge is reduced allowing the filaments to slide. Movement of the myosin head during low load shortening creates negative stress that causes ADP to be quickly released from the myosin head followed by the binding of further ATP which turns the myosin motor of causing the cross-bridge to break and fall into a super-relaxed state. However, during low load high shortening speeds the cross-bridges are forcibly broken prematurely before ADP-release and ATP-binding generating heat and an accumulation of detached cross-bridges with thweir myosin motors in the super-relaxed off-state. When tension redevelops after the shortening there is extra ATP splitting. During a high force contraction ADP is slowly released but with no movement at the myosin head and depleted ATP preventing its binding the cross-bridge doesn’t break.
Stretching out and winding up of cross-bridge stiffness and titin in the lengthening phase enables it to recoil and fall slack on the shortening phase. This decreases cross-bridge and titin stiffness to increase the slack length in intrafusal fibers and tolerance to subsequent stretching which reduces muscle spindle firing (Macefield & Knellwolf 2018). If, when in a resting state gamma firing doesn’t isometrically contract intrafusal fibers to remove the slack created by the prior stretch, or, not enough time is given enough to allow cross-bridges to return to their old steady predictable rate of spontaneous formation and breakage, further stretching will result in a different instantaneous muscle spindle firing responses (Blum et al 2020).
2. Dynamic cyclic stretching. Dynamic stretching resets stable cross-bridges. Producing an initial (<rapid) stretch straining the cross-bridges results in muscle spindles responding with higher levels of action potentials. Even though the fascicles (and spindles) are lengthened on subsequent repetitions of the stretch these initially strained cross-bridges detach so that activity in the spindle reduces (Haftel et al 2004), especially when there are shorter intervals between the stretches (Abbott et al 2024). So not only do these rapid, large-amplitude movements break any pre-existing stable cross-bridges between actin and myosin filaments within the intrafusal fibres it also creates a yielding of the elastic titin (Morgan et al 1984). But even though the cross-bridges overlap is being reduced as they get pulled apart at this longer length passive stiffness in the intrafusal muscle fibers still develops as stretching titin strengthens the cross-bridges in this lengthened state (Morgan et al 1984 & Gregory et al 1998). On rapid passive shortening, titin recoils and fulls slack (Monry et al 2012) and the intrafusal fibers, being unable to ‘keep up’ with the rate of passive shortening, also fall slack to reduce the passive tension in the intrafusal muscle fibers and reduce spindle firing (Blum et al 2020). Cyclic stretching increases range of motion to reduce muscle stiffness and tension by breaking the actin-myosin cross-bridges allowing the muscle fibers to lengthen (Maeda et al 2017) and reduce viscosity by rapidly redistributing the more mobile constituents such as the polysaccharides and water within the collagen framework (McNair et al 2001).
3. Static stretching. The epimysium is continuous with the outer paratenon (Peña-Amaro 2021), the perimysium, as well as embedding intrafusal fibers (Omstead et al 2023), is also continuous with the musculotendinous junction, tendons and aponeuroses and the endomysium is continuous with perimysium at its tendon attachments (Peña-Amaro 2021). Also, at the musculotendinous junction microtendons from the lateral side of each muscle fiber and its endomysium stabilises the muscle fibers, as each microtendon joins the perimysium and epimysium to forms the muscle’s tendon (Järvinen et al 2002).
During stretch increasing elasticity and compliance of this muscle-musculotendinous-tendon complex reduces displacement of the muscle’s fascicles and spindles, and, in turn mechanosensory feedback. In older adults where more compliant tendons reduce muscle spindle activation and proprioceptive feedback negatively affecting movement and posture (Abbott et al 2024). Conversely increased compliance during contraction means the muscle can produce the most force at shorter lengths before pulling on and stretching the aponeuroses and tendons. Static stretching reduces muscle stiffness to produces greater increases in range of motion than cyclic stretching by relaxing the muscle to increase flexibility in the aponeurosis and the connective tissue, i.e. the endomysium, perimysium, and epimysium (Maeda et al 2017) which can allow greater elongation of the muscle fibers. Although elongation of the fascicle contributes more than elongation of the free tendon to lengthening of the musculotendinous junction (Lévenéz et al 2024), to reduce musculotendinous stiffness stretches must be held for 240 secs at a high intensity (Kamandulis et al 2024).
Whilst mechanisms to reduce muscle tension can be advantageous it can be detrimental to reduce the maximal force under which a muscle is able to stabilise a given limb position (isometric condition). This results in premature eccentric contraction (Bittmann et al 2023) resulting in muscle pain from the stimulation of muscle spindles (Sas et al 2023) and damages as muscles become ineffcient in trying to decelerate against external loads (Bittmann et al 2023).
Mitochondria & sensory processing
Function of mitochondria
Mitochondria regulate inflammatory responses and neuronal functions. Having a higher energetic demand in comparison to other cell types neurones rely heavily on glucose metabolism and fatty acids in mitochondrial respiration to synthesise ATP. This is especially so for primary afferent dorsal root ganglion neurones that have long processes and therefore a high protein turnover which is required for molecules to be produced and transported by axoplasmic flow in sufficient quantities over long distances. Despite the cell bodies of the DRG neurones being large, the majority of the mitochondria are located along the axonal processes of these neurones, although the axon hillock, that, along with the axonal initial segment, determines the passage of action potentials to the central terminals of the sensory neurones in the dorsal horn, possesses larger mitochondria. Whilst mitochondria increase with cell size during development they subsequently decrease with age and can change size and morphology according to the energy demand (Haberberger et al 2023).
Mitochondrial reactive oxygen species (mtROS) is produced as a byproduct of respiration when producing ATP (Silva et al 2022). Ninety eight percent of inhaled oxygen is utilised by the mitochondria all over the body and one to two percent of total daily oxygen consumption goes to producing reactive oxygen species (ROS) (Kausar et al 2018). Ninety percent of this cellular reactive oxygen species (ROS) being generated by the mitochondria (Silva et al 2022).
MtROS is important for cell signalling and innate host defence against pathogens by initiating inflammatory responses e.g. inflammasome (NLRP3) (Silwal et al 2020). However, when left unchecked, from either (i) ROS overproduction (e.g. increases mitochondrial concentrations of calcium, refer below, or exposure to certain inflammatory agents), or (ii) decreased antioxidant defence, oxidative stress ensues causing damage to the mitochondria, neuroinflammation & neurodegeneration (Silva et al 2022).
Mitochondria (along with the endoplasmic reticulum) also serves as intracellular calcium reservoirs as they regulate intracellular calcium concentration. Under normal physiological conditions the mitochondria should take up more calcium than it releases. This is because calcium is a key regulator of mitochondrial function and stimulates ATP synthesis allowing the mitochondria to produce higher ATP outputs to meet the cellular ATP demands. However, in pathology, high mitochondrial calcium concentrations, along with the accumulation of oxidants and the depletion of adenine nucleotides, makes the mitochondria more porus to molecules. This leads to (i) mitochondrial swelling and death, (ii) stimulates production of mtROS leading to oxidative stress and (iii) releases Cytochrome C from the mitochondria resulting in apoptosis (Brookes et al 2004). It is this extracellular release of Cytochrome C, mtROS or mitochondrial DNA that triggers the inflammatory response that regulates neuronal activity and pain. This is how drugs that inhibit mitochondrial calcium uptake in the spinal cord and neurones reduces pain (Silva et al 2022).
Mitochondria also controls the release of neurotransmitters, neuronal excitability, signalling and plasticity which is also another way through which modulating mitochondrial functions in sensory neurones can reduce hyperalgesia (Silva et al 2022).
Inflammation & mitochondrial respiration
Exposure of neurones to inflammatory mediators alter mitochondrial respiration in neurones. Some inflammatory agents promote mitochondrial respiration which increases ATP production, but as a byproduct of this, produces mtROS which damages the mitochondria and promotes neuroinflammation and neurodegeneration. Increases in ATP production can sustain inflammatory responses (refer ‘figure five’). Other inflammatory mediators reduce mitochondrial respiration which decreases ATP production (refer ‘figure six’) (Silva et al 2022).
weFigure five: the effects of inflammatory agents on increasing oxidative stress in the mitochondria (Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N 2023. Mitochondria and sensory processing in inflammatory and neuropathic pain).raes
Figure six: the effects of inflammation on mitochondrial deficiency effecting the lactate/pyruvate ratio (increasing lactate) and the NAD+/NADH ratio (increasing NADH) (Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N 2023. Mitochondria and sensory processing in inflammatory and neuropathic pain).
Mitochondrial mitophagy & biogenesis
The balance of mitochondrial mitophagy and biogenesis is critical for maintaining proper cellular functions. Biogenesis is driven by decreased energy (ATP) production (Silva et al 2022), oxidative damage to the mitochondria from mtROS (that is a by-product of ATP production) and endurance training and low temperature (4deg C). In response to the need for ATP, and mitochondrial repair from oxidative stress, biogenesis produces new functional mitochondria by triggering their autoreplication from pre-existing mitochondria (Jornayvaz & Shulman 2010).
Consequentially, increase mtROS production or mitochondrial calcium concentration leads to an inflammatory response which leads to the elimination of damaged mitochondria via mitophagy leaving only healthy mitochondria restoring mtROS levels and calcium concentration. Dysfunctional (increased) mitophagy, or defective (decreased) mitochondrial biogenesis affects sensory processing by producing an overall less efficient mitochondrial pool (Silva et al 2022).
Inflammation triggers mitography and inhibits biogenesis, resulting in more mitochondria being destroyed than being produced leading to an overall depletion of mitochondria and impaired ATP and increased mtROS production. This can account for pain in inflammatory conditions, such as osteoarthritis and neuropathic pain, that trigger dysfunctional mitophagy and biogenesis. Decreased mitochondrial biogenesis also reduces the total pool of mitochondria in brain neurons leading to neuroinflammation and neurodegeneration as seen in Parkinson's and Alzheimer's disease (Silva et al 2022).
Mitochondrial fusion and fission
Mitochondrial fusion is when adjacent mitochondria merge permitting a mixture of mitochondrial content it is required for embryonic development and for cell survival at later stages in development. Mitochondrial fusion generally promotes a more efficient and interconnected mitochondrial network as in response to low levels of oxidative stress DNA mutations in one mitochondria can fuse, and be compensated for with DNA from another mitochondria, thereby rescuing the impaired mitochondria. This helps maximise the new mitochondria’s oxidative capacity and in turn its ATP production under stressful conditions (Youle & Bilek 2016).
However, under high levels of oxidative stress badly damaged mitochondria will contaminate other mitochondria if they are allowed to rejoin the mitochondrial network. In such a case mitochondrial fission segregates the damaged parts of mitochondria and targets them for elimination by mitophagy. Therefore, mitochondrial fission by sifting out damaged parts of mitochondria and targeting them for elimination is essential for mitochondrial mitophagy (Youle & Bilek 2016) and biogenesis (Silva et al 2022) in order to create an efficient mitochondrial pool.
Inflammatory conditions, by disturbing ATP production, promoting oxidative stress and calcium influx into the mitochondria, are more likely to induce mitochondrial fission resulting in fragmented mitochondria, impaired energy production, and disrupted mitochondrial homeostasis. In turn, changes in mitochondrial function from mitochondrial fission has a neurotoxic effect, as seen in Alzheimer’s, and promotes the release of inflammatory mediators that sensitise or activate the nervous system contributing to pain (Silva et al 2022) (refer ‘figure seven’). Mitochondrial fusion can attempt to compensate for the inflammatory induced breaking up of the mitochondria that triggers mitophagy to eliminate the damaged mitochondria (Youle & Bilek 2016).
Figure seven: Inflammation & mitochondrial fission (Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N 2023. Mitochondria and sensory processing in inflammatory and neuropathic pain).
Metabolic dysfunction in fibromyalgia & RA
Markers of mitochondrial dysfunction and mitochondrial related genetic modifications are present in rheumatic patients, for instance, RA and fibromyalgia patients express reduced intracellular (e.g. blood, skin) ATP levels and elevated mtROS levels (Silva et al 2022).
References
Macionis V. (2023). Chronic pain and local pain in usually painless conditions including neuroma may be due to compressive proximal neural lesion.
Pirri C, Pirri N, Guidolin D, Macchi V, Porzionato A, De Caro R, Stecco C. (2023). Ultrasound Imaging of Thoracolumbar Fascia Thickness: Chronic Non-Specific Lower Back Pain versus Healthy Subjects; A Sign of a "Frozen Back"?
Kwak HH, Ko SJ, Jung HS, Park HD, Chung IH, Kim HJ (2003). Topographic anatomy of the deep temporal nerves, with references to the superior head of lateral pterygoid.
Teixeira MJ (2016). Concept of acute neuropathic pain. The role of nervi nervorum in the distinction between acute nociceptive and neuropathic pain
Wiberg A, Ng M, Schmid AB, Smillie RW, Baskozos G, Holmes MV, Künnapuu K, Mägi R, Bennett DL, Furniss D. (2019) A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome.
Gaughran G (1957). FASCIAE OF THE MASTICATOR SPACE
BARKER B & DAVIES P (1972). THE APPLIED ANATOMY OF THE PTERYGOMANDIBULAR SPACE
Topp KS & Boyd BS (2006). Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice.
Kitamura T, Takagi K, Yamaga M, Morisawa K. (1995). Brachial plexus stretching injuries: microcirculation of the brachial plexus.
Chwei-Chin Chuang D, Fang F, Nai-Jen Chang T, Chuieng-Yi Lu J. (2016). Thoracic Outlet Syndrome: Past and Present-88 Surgeries in 30 Years at Chang Gung.
Silva Santos Ribeiro P, Willemen HLDM, Eijkelkamp N. (2022). Mitochondria and sensory processing in inflammatory and neuropathic pain.
Menovsky, T. (1999). Laser-assisted nerve repair. An experimental study
Baselgia LT, Bennett DL, Silbiger RM, Schmid AB (2017). Negative Neurodynamic Tests Do Not Exclude Neural Dysfunction in Patients With Entrapment Neuropathies
Li Z, Jiang Z, Lu L, Liu Y. (2023). Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems.
Bove GM. (2008). Epi-perineurial anatomy, innervation, and axonal nociceptive mechanisms.
Foran IM, Hussey V, Patel RA, Sung J, Shah SB. (2018). Native paraneurial tissue and paraneurial adhesions alter nerve strain distribution in rat sciatic nerves
Silwal P, Kim JK, Kim YJ, Jo EK (2020). Mitochondrial Reactive Oxygen Species: Double-Edged Weapon in Host Defense and Pathological Inflammation During Infection
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. (2004). Calcium, ATP, and ROS: a mitochondrial love-hate triangle
Kausar S, Wang F, Cui H. (2018). The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases
Del-Blanco-Muñiz JA, Martín-Vera D, Sosa-Reina MD, Trinidad-Morales A, de-la-Plaza-San-Frutos M, Sánchez-Sierra A. Cervical Impairments in Subjects with Chronic Migraine: An Observational Study
Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. (2002). Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents
Jornayvaz FR, Shulman GI. (2010) Regulation of mitochondrial biogenesis
Youle RJ, van der Bliek AM. (2016). Mitochondrial fission, fusion, and stress
Andreeva D, Murashova L, Burzak N, Dyachuk V. (2022). Satellite Glial Cells: Morphology, functional heterogeneity, and role in pain
Krock E, Morado-Urbina CE, Menezes J, Hunt MA, Sandström A, Kadetoff D, Tour J, Verma V, Kultima K, Haglund L, Meloto CB, Diatchenko L, Kosek E, Svensson CI. (2023). Fibromyalgia patients with elevated levels of anti-satellite glia cell immunoglobulin G antibodies present with more severe symptoms.
Bogduk N (1981). An anatomical basis for the Neck-Tongue Syndrome
Haberberger RV, Barry C, Dominguez N, Matusica D. (2019). Human Dorsal Root Ganglia.
Haberberger RV, Kuramatilake J, Barry CM, Matusica D. (2023). Ultrastructure of dorsal root ganglia.
Weerasuriya A & Mizisin A (2011). The Blood-Nerve Barrier: Structure and Functional Significance
Berciano J (2018). Axonal pathology in early stages of Guillain-Barré syndrome.
Aliyarbayova A, Mehraliyeva G, Sadiqova G, Nacafova T, Mansimov A (2022). Ultrastructural peculiarities of perineurial cells of capsular elements of dorsal root ganglia. Animal model of study
Martin SL, Reid AJ, Verkhratsky A, Magnaghi V, Faroni A. (2019). Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in inflammation, cell death and nociception.
Fanton S, Menezes J, Krock E, Sandström A, Tour J, Sandor K, Jurczak A, Hunt M, Baharpoor A, Kadetoff D, Jensen KB, Fransson P, Ellerbrock I, Sitnikov R, Svensson CI, Kosek E. (2023). Anti-satellite glia cell IgG antibodies in fibromyalgia patients are related to symptom severity and to metabolite concentrations in thalamus and rostral anterior cingulate cortex
Brandl A, Egner C, Reer R, Schmidt T, Schleip R. (2022). Associations between Deformation of the Thoracolumbar Fascia and Activation of the Erector Spinae and Multifidus Muscle in Patients with Acute Low Back Pain and Healthy Controls: A Matched Pair Case-Control Study
Fede C, Petrelli L, Guidolin D, Porzionato A, Pirri C, Fan C, De Caro R, Stecco C. (2021). Evidence of a new hidden neural network into deep fasciae
Stecco A, Giordani F, Fede C, Pirri C, De Caro R, Stecco C. (2023). From Muscle to the Myofascial Unit: Current Evidence and Future Perspectives.
Omstead KM, Williams J, Weinberg SM, Marazita ML, Burrows AM. (2023). Mammalian facial muscles contain muscle spindles.
Partanen JV, Lajunen HR, Liljander SK. (2023). Muscle spindles as pain receptors
Johnson LR, Battle AR, Martinac B. (2019). Remembering Mechanosensitivity of NMDA Receptors
Tai Y, Woods EL, Dally J, Kong D, Steadman R, Moseley R, Midgley AC. (2021). Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin FibrosisStecco A, Stecco C, Raghavan (2014). Peripheral Mechanisms Contributing to Spasticity and Implications for Treatment
Järvinen TA, Józsa L, Kannus P, Järvinen TL, Järvinen M. (2002). Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study.
Stecco C, Fede C, Macchi V, Porzionato A, Petrelli L, Biz C, Stern R, De Caro R. (2018). The fasciacytes: A new cell devoted to fascial gliding regulation.
Pratt RL. (2021). Hyaluronan and the Fascial Frontier.
Fan C, Pirri C, Fede C, Guidolin D, Biz C, Petrelli L, Porzionato A, Macchi V, De Caro R, Stecco C. (2021). Age-Related Alterations of Hyaluronan and Collagen in Extracellular Matrix of the Muscle Spindles.
Nichols TR. (2017). Distributed force feedback in the spinal cord and the regulation of limb mechanics
Maas H, Noort W, Smilde HA, Vincent JA, Nardelli P, Cope TC. (2022). Detection of epimuscular myofascial forces by Golgi tendon organs.
Amir A, Kim S, Stecco A, Jankowski MP, Raghavan P. (2022). Hyaluronan homeostasis and its role in pain and muscle stiffness.
Smilde HA, Vincent JA, Baan GC, Nardelli P, Lodder JC, Mansvelder HD, Cope TC, Maas H. (2016). Changes in muscle spindle firing in response to length changes of neighboring muscles
Torell F, Franklin S, Franklin DW, Dimitriou M. (2023). Assistive Loading Promotes Goal-Directed Tuning of Stretch Reflex Gains
Khan MN, Cherukuri P, Negro F, Rajput A, Fabrowski P, Bansal V, Lancelin C, Lee TI, Bian Y, Mayer WP, Akay T, Müller D, Bonn S, Farina D, Marquardt T (2022). ERR2 and ERR3 promote the development of gamma motor neuron functional properties required for proprioceptive movement control
Dimitriou M. (2022). Human muscle spindles are wired to function as controllable signal-processing devices
Linke WA. (2023). Stretching the story of titin and muscle function.
Tomalka A, Weidner S, Hahn D, Seiberl W, Siebert T. (2021). Power Amplification Increases With Contraction Velocity During Stretch-Shortening Cycles of Skinned Muscle Fibers
Lin Z, Yang J, Lin Y, Cheng Y, Hung C, Chen C, Chou L (2021). Effect of kinesio taping on hand sensorimotor control and brain activity
Reed WR, Pickar JG. (2015). Paraspinal Muscle Spindle Response to Intervertebral Fixation and Segmental Thrust Level During Spinal Manipulation in an Animal Model.
Noureddine M, Gehmlich K. (2023). Structural and signaling proteins in the Z-disk and their role in cardiomyopathies.
Monroy JA, Powers KL, Gilmore LA, Uyeno TA, Lindstedt SL, Nishikawa KC. (2012). What is the role of titin in active muscle?
Joumaa V, Herzog W. (2014). Calcium sensitivity of residual force enhancement in rabbit skinned fibers
Sas D, Gaudel F, Verdier D, Kolta A. (2024). Hyperexcitability of muscle spindle afferents in jaw-closing muscles in experimental myalgia: Evidence for large primary afferents involvement in chronic pain.
Bittmann FN, Dech S, Schaefer LV. (2023). How to Confuse Motor Control: Passive Muscle Shortening after Contraction in Lengthened Position Reduces the Muscular Holding Stability in the Sense of Adaptive Force
Morgan D.L., Prochazka A., Proske U. (1984). The After-Effects of Stretch and Fusimotor Stimulation on the Responses of Primary Endings of Cat Muscle Spindles
Gregory JE, Wise AK, Wood SA, Prochazka A, Proske U. (1998). Muscle history, fusimotor activity and the human stretch reflex.
Muthu P, Talent JM, Gryczynski I, Borejdo J. (2008). Cross-bridge duty cycle in isometric contraction of skeletal myofibrils.
Blum KP, Campbell KS, Horslen BC, Nardelli P, Housley SN, Cope TC, Ting LH. (2020). Diverse and complex muscle spindle afferent firing properties emerge from multiscale muscle mechanics.
Hessel AL, Ma W, Mazara N, Rice PE, Nissen D, Gong H, Kuehn M, Irving T, Linke WA. (2022). Titin force in muscle cells alters lattice order, thick and thin filament protein formation
Haftel VK, Bichler EK, Nichols TR, Pinter MJ, Cope TC. (2004). Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat
Abbott EM, Stephens JD, Simha SN, Wood L, Nardelli P, Cope TC, Sawicki GS, Ting LH. (2024). Attenuation of muscle spindle firing with artificially increased series compliance during stretch of relaxed muscle.
Peña-Amaro J (2021). The musculotendinous transition of the extracellular matrix
Maeda N, Urabe Y, Tsutsumi S, Sakai S, Fujishita H, Kobayashi T, Asaeda M, Hirata K, Mikami Y, Kimura H. (2017). The Acute Effects of Static and Cyclic Stretching on Muscle Stiffness and Hardness of Medial Gastrocnemius Muscle
Li YY, Ji SF, Fu XB, Jiang YF, Sun XY. (2024). Biomaterial-based mechanical regulation facilitates scarless wound healing with functional skin appendage regeneration.
Kamandulis S, Werasirirat P, Namsawang J, Singhasoot N, Snieckus A, Muanjai P. (2024). Acute Effects of Combined and Distinctive Stretching, Foam Rolling, and Eccentric Exercise in Young Men with Hamstring Tightness.
McNair PJ, Dombroski EW, Hewson DJ, Stanley SN. (2001). Stretching at the ankle joint: viscoelastic responses to holds and continuous passive motion.
Jarosch R. (2008). Large-scale models reveal the two-component mechanics of striated muscle
DuVall MM, Jinha A, Schappacher-Tilp G, Leonard TR, Herzog W. (2017). Differences in titin segmental elongation between passive and active stretch in skeletal muscle.
Fenwick AJ, Wood AM, Tanner BCW. (2017). Effects of cross-bridge compliance on the force-velocity relationship and muscle power output
Karatzaferi C, Chinn MK, Cooke R. (2005). The force exerted by a muscle cross-bridge depends directly on the strength of the actomyosin bond
Linari M, Brunello E, Reconditi M, Fusi L, Caremani M, Narayanan T, Piazzesi G, Lombardi V, Irving M. (2015). Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments
Fusi L, Brunello E, Yan Z, Irving M. (2016). Thick filament mechano-sensing is a calcium-independent regulatory mechanism in skeletal muscle
Morotti I, Caremani M, Marcello M, Pertici I, Squarci C, Bianco P, Narayanan T, Piazzesi G, Reconditi M, Lombardi V, Linari M. (2024). An integrated picture of the structural pathways controlling the heart performance.
Squarci C, Bianco P, Reconditi M, Pertici I, Caremani M, Narayanan T, Horváth ÁI, Málnási-Csizmadia A, Linari M, Lombardi V, Piazzesi G. (2023). Titin activates myosin filaments in skeletal muscle by switching from an extensible spring to a mechanical rectifier
Squarci C, Bianco P, Reconditi M, Pertici I, Caremani M, Narayanan T, Horváth ÁI, Málnási-Csizmadia A, Linari M, Lombardi V, Piazzesi G. (2021). Titin switches from an extensible spring to a mechanical rectifier upon muscle activation
Hanft LM, Greaser ML, McDonald KS. (2015). Titin-mediated control of cardiac myofibrillar function
Linari M, Piazzesi G, Pertici I, Dantzig JA, Goldman YE, Lombardi V. (2020). Straightening Out the Elasticity of Myosin Cross-Bridges.
Sudarshi Premawardhana DM, Zhang F, Xu J, Gage MJ. (2020). The Poly-E motif in Titin's PEVK region undergoes pH dependent conformational changes
Yan Y, Antolin N, Zhou L, Xu L, Vargas IL, Gomez CD, Kong G, Palmisano I, Yang Y, Chadwick J, Müller F, Bull AMJ, Lo Celso C, Primiano G, Servidei S, Perrier JF, Bellardita C, Di Giovanni S (2025). Macrophages excite muscle spindles with glutamate to bolster locomotion